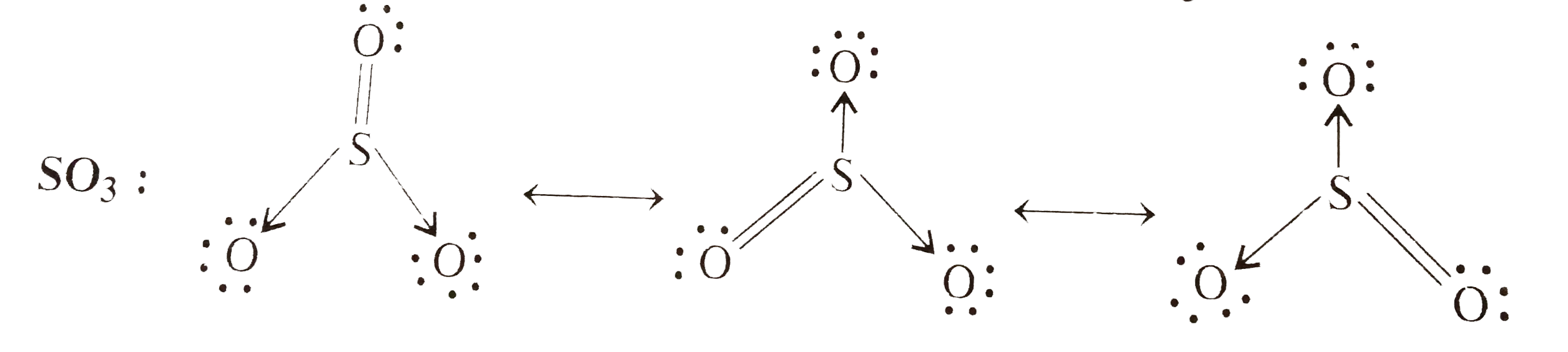
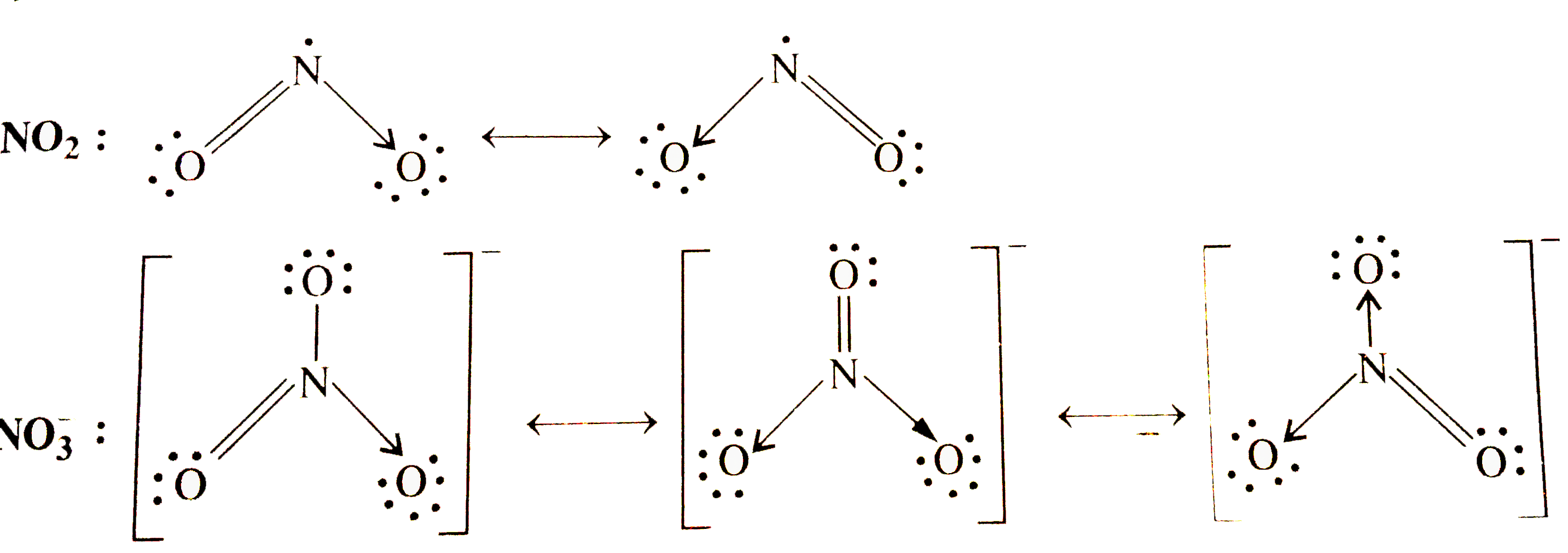
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the resonance structures for NO2
Text Solution
|
- Give the resonating structures of NO2 and N2O5 .
Text Solution
|
- How many resonating structures can be drawn for NO2?
Text Solution
|
- Write resonating structure of the compound
Text Solution
|
- Write the resonance structure of CO2 .
Text Solution
|
- NO2 तथा N2O5 की अनुनादी संरचनाओं को लिखिए।
Text Solution
|
- NO2,NO3^(-) Write resonance structures.
Text Solution
|
- Give the resonating structures of NO2 and N2O5
Text Solution
|
- Give the resonating structures of NO2 and N2O5 .
Text Solution
|