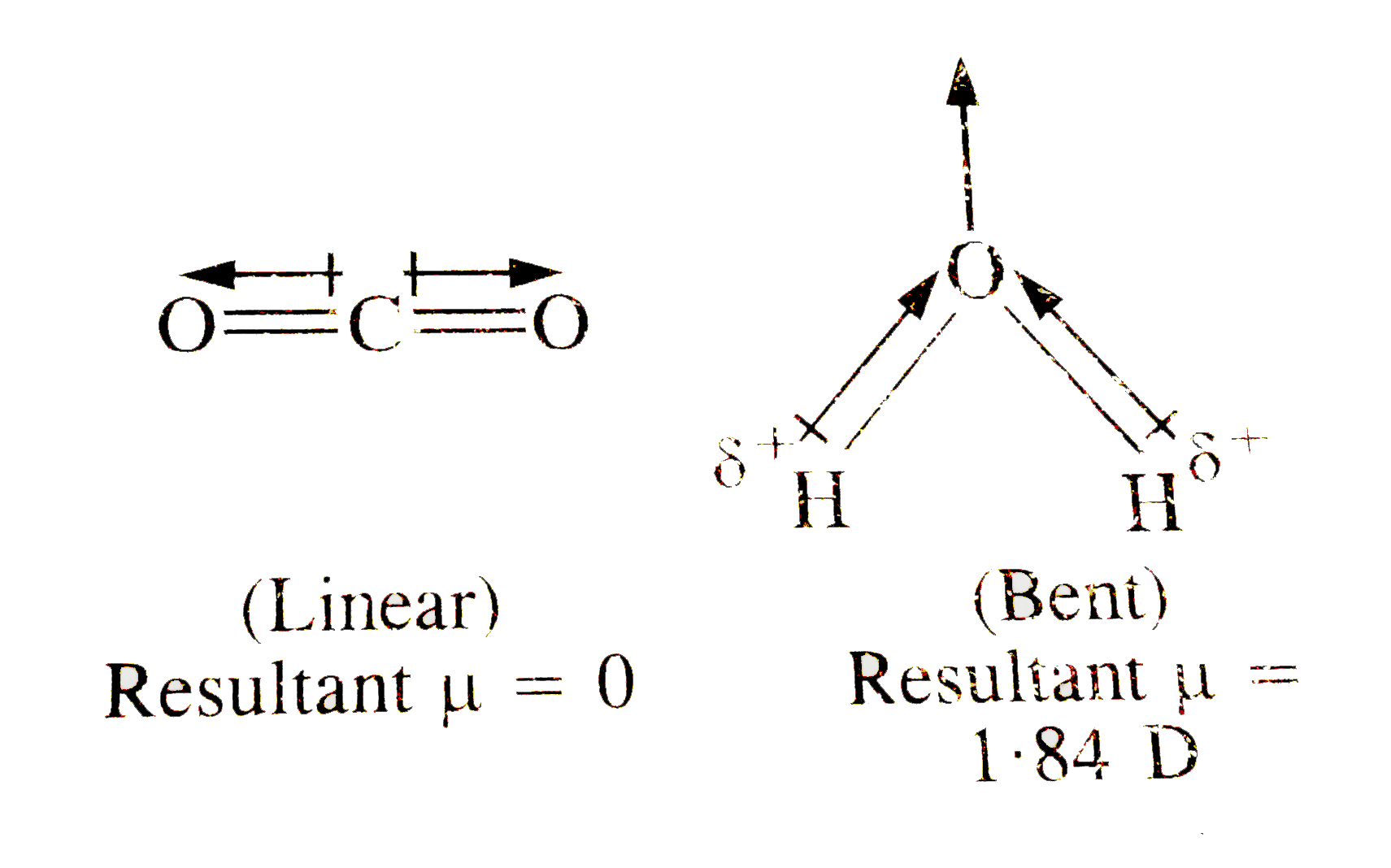Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Although both CO(2) and H(2)O are triatomic molecules, the shape of H(...
Text Solution
|
- The net dipole moment of H(2)O molecule is
Text Solution
|
- H(2)O molecule is a triatomic molecule but its geometry is not linear....
Text Solution
|
- हालांकि CO(2) तथा H(2)O दोनों त्रिप्रमाणुक अणु हैं, परन्तु H(2)O अणु क...
Text Solution
|
- CO(2) व H(2)O दोनों त्रिपरमाणविक अणु हैं परंतु इनके द्विध्रुव आघुर्ण म...
Text Solution
|
- Although both CO(2) & H(2)O are triatomic molecules, the shape of H(2)...
Text Solution
|
- हालॉंकि CO(2)" तथा "H(2)O दोनों त्रिपरमाणुक अणु है , परन्तु H(2)O अणु ...
Text Solution
|
- CO(2) " and " H(2) O both are triatomic molecule but their dipole mom...
Text Solution
|
- CO(2) and H(2)O both are triatomic molecules but their dipole moment v...
Text Solution
|