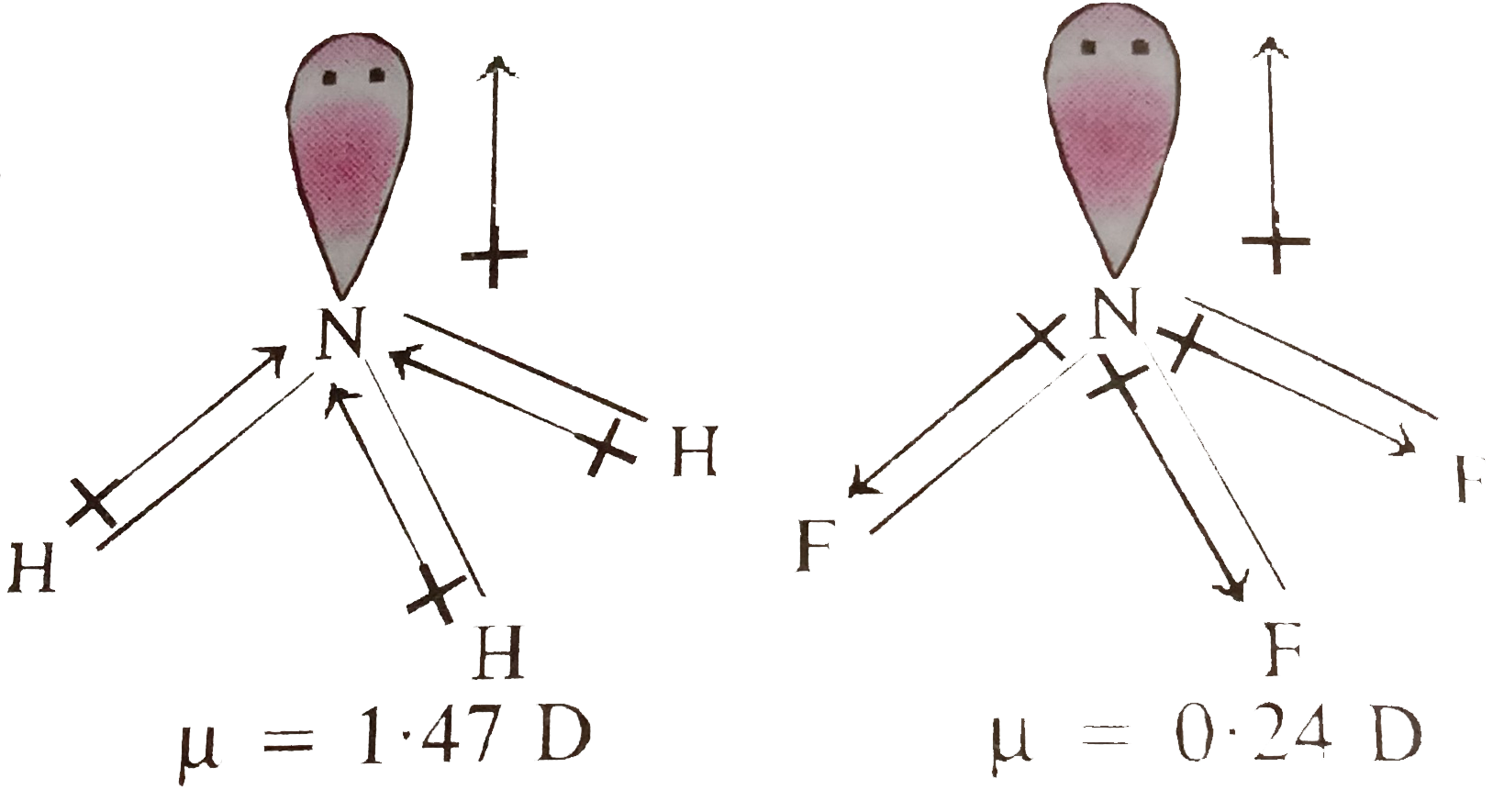Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Both NH(3) and NF(3) have identical shapes and same state of hybridisa...
Text Solution
|
- How many molecules among the following have zero dipole moment NH(3),B...
Text Solution
|
- Assertion : Molecular having different hybridisation can have same sha...
Text Solution
|
- NF(3) " and " NH(3) are both pyramidal but differ widely in dipole mom...
Text Solution
|
- N व F के बीच विघुत ऋणात्मकता का अन्तर N और H परमाणुओं की अपेक्षा उच्च...
Text Solution
|
- Statement-1 : Dipole moment of NF(3) is less than that of NH(3) . Stat...
Text Solution
|
- Statement I : Bond angle of BF(3) " and " NF(3) are different State...
Text Solution
|
- N तथा H के बीच विद्युत - ऋणात्मकता के अंतर से अधिक अंतर N तथा F के ...
Text Solution
|
- Assertion : Dipole moment of NF(3) is less than that of NH(3). Reaso...
Text Solution
|