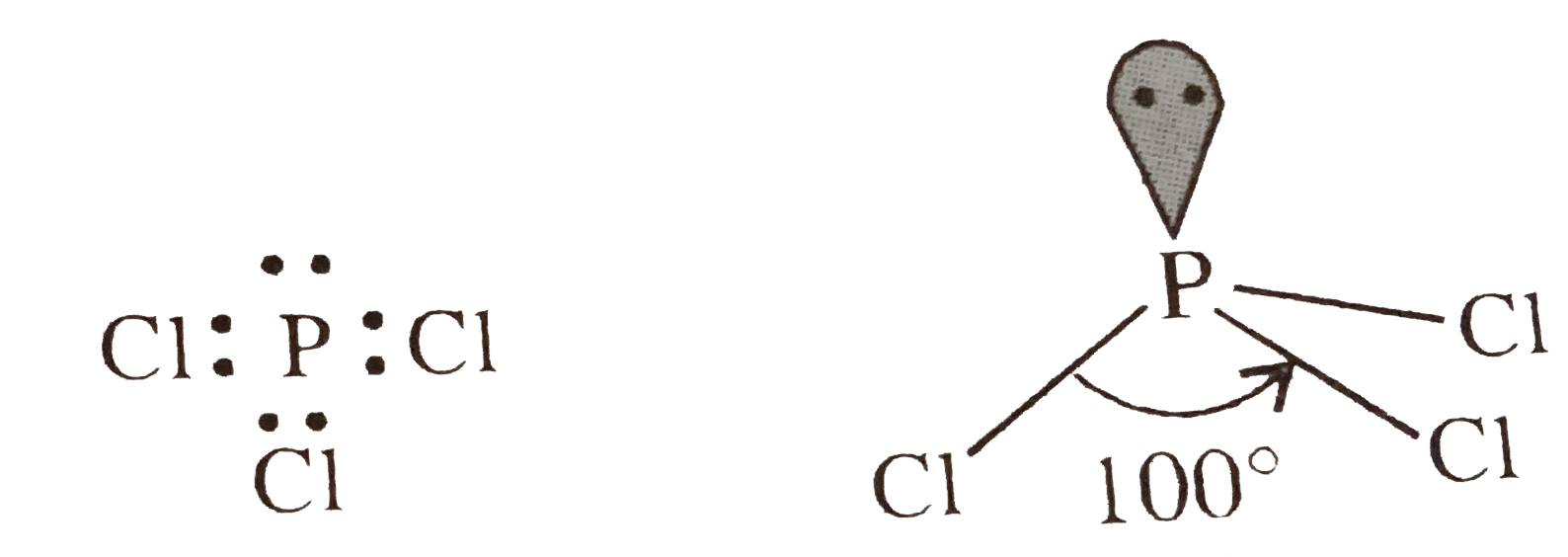Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
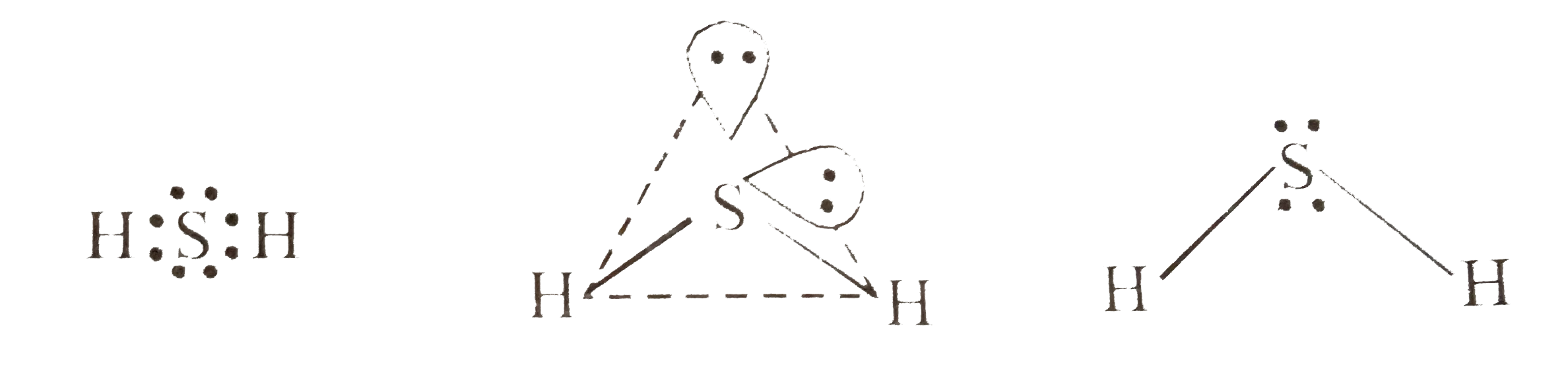
- Explain the non-linear shape of H(2)S and non-planar shape of PCl(3) u...
Text Solution
|
- Interpret the non-linear shape of H(2)S molecule and non-planar shape ...
Text Solution
|
- Interpret non-linear shape of H(2)S and non-planar shape of PCl(3) on ...
Text Solution
|
- The shape of ClO3^- according to valence shell electron pair repulsion...
Text Solution
|
- Although the number of bond pair of electrons in the valence shell of ...
Text Solution
|
- संयोजकता कोष इलेक्ट्रॉन युग्म प्रतिकर्षण सिद्धांत (VSEPR theory का प्र...
Text Solution
|
- Write any three main features of the VSEPR theory.
Text Solution
|
- Let us describe the following atomic shapes using the Valence Carr ele...
Text Solution
|
- Let us describe the following atomic shapes using the Valence Carr ele...
Text Solution
|