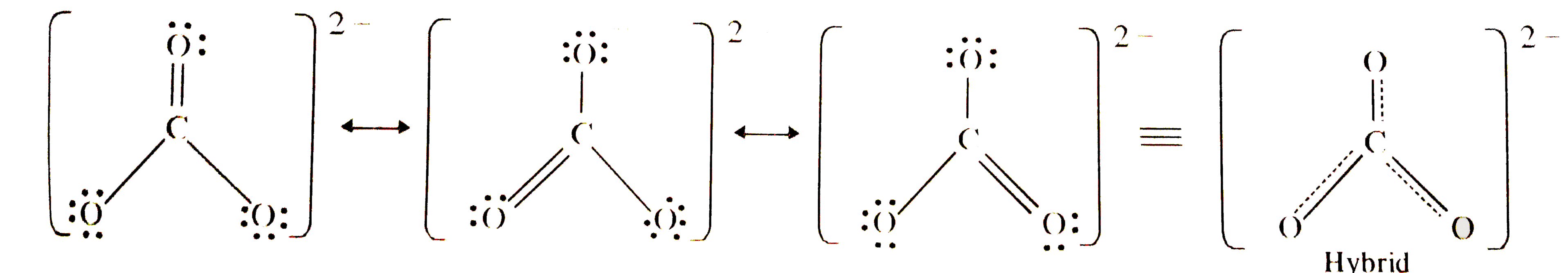Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain why CO(3)^(2-) ion cannot be represented by a single Lewis str...
Text Solution
|
- Write the Lewis dot structure of CO(3)^(2-) ion .
Text Solution
|
- Explain the structure of CO(3)^(2-) ion in terms of resonance (b) Ex...
Text Solution
|
- The structure of ozone can best be represented by
Text Solution
|
- Write the Lewis dot structure of CO(3)^(2-) ion .
Text Solution
|
- निम्नलिखित अणुओं तथा आयनों की लुईस संरचनाएँ लिखिए । CO(3)^(-2)
Text Solution
|
- लुईस संकेतों के द्वारा दर्शायी गई आणविक संरचना लुईस संरचना कहलाती है।
Text Solution
|
- Explain resonance with reference to carbonate ion. Lewis structur...
Text Solution
|
- Draw Lewis structures for the following molecules and ions : AlI(3)," ...
Text Solution
|