Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
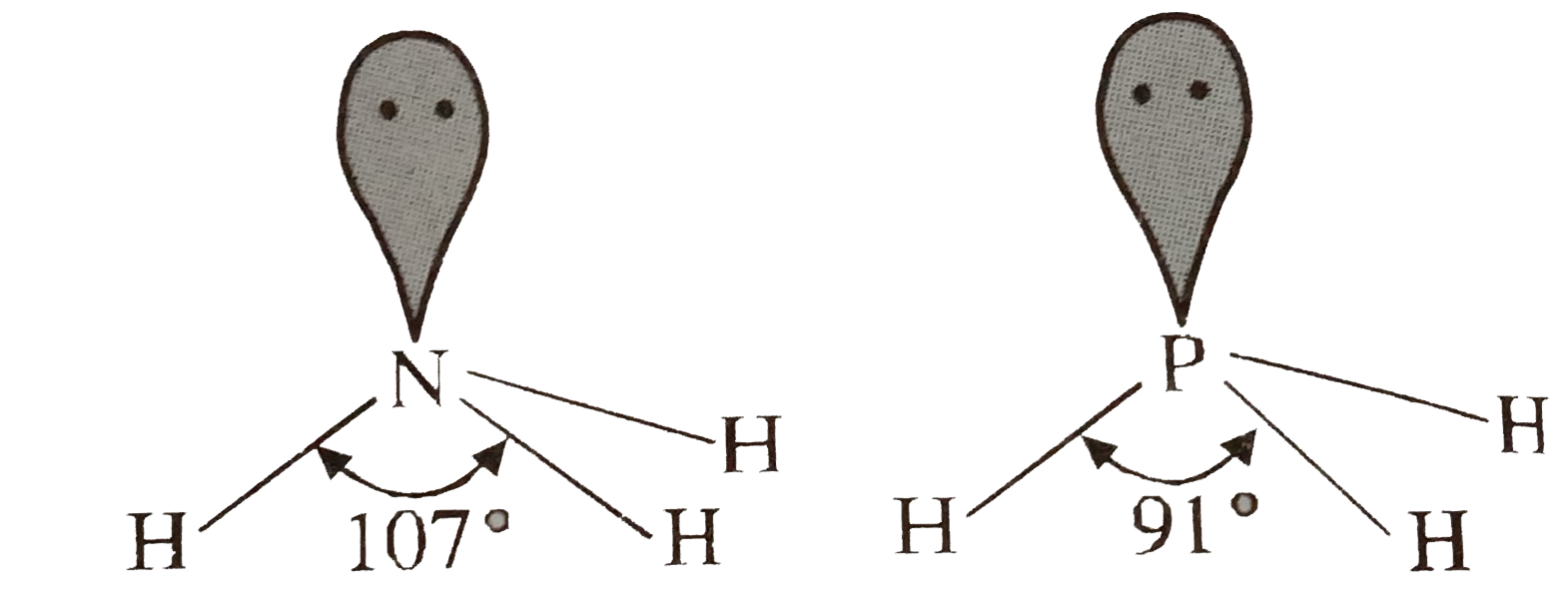
- Bond angle in NH(3) is more than in PH(3). Explain.
Text Solution
|
- Decreasing order of bond angle of (NH(3), PH(3), AsH(3)) is
Text Solution
|
- Bond angle in PH(4)^(+) is higher than that in PH(3).Why ?
Text Solution
|
- Why is bond angle in PH(4)^(+) ion higher than in PH(3) ?
Text Solution
|
- Both PH(3) and NH(3) are Lewis bases, but basic strength of PH(3) is l...
Text Solution
|
- NH(3) has higher proton affinity than PH(3). Explain. Or NH(3) is more...
Text Solution
|
- PH(3) का आबन्ध कोण NH(3) की अपेक्षाकृत छोटा क्यों होता है?
Text Solution
|
- PH(3) से PH(4)^(+) का अबंध कोण अधिक है, क्यों ?
Text Solution
|
- NH(3) का क्वथनांक PH(3) से अधिक होने का कारण अमोनिया अणुओं के मध्य ......
Text Solution
|