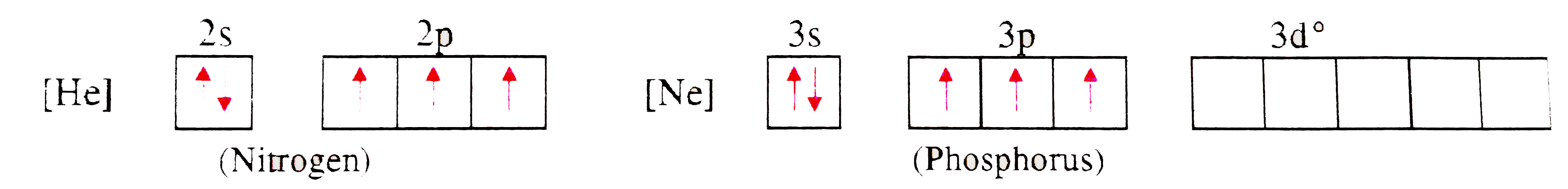
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain : NCl5 does not exists but PCl5 exists.
Text Solution
|
- PCl5 and PH3 exist but PH5 does not because
Text Solution
|
- Why NI5 does not exist While PCl5 exists?
Text Solution
|
- PCl5 exists but NCl5 does not because :
Text Solution
|
- Amongst the following the total number of species which does/do not ex...
Text Solution
|
- PCl3 and PCl5 both exist but only PH3 exists while PH5 does not exist ...
Text Solution
|
- PCl5 exists but NCl5 does not exist why
Text Solution
|
- NCl5 does not exist but PCl5 exists. Why?
Text Solution
|
- PCl5 exists but NCl3 does not because:
Text Solution
|