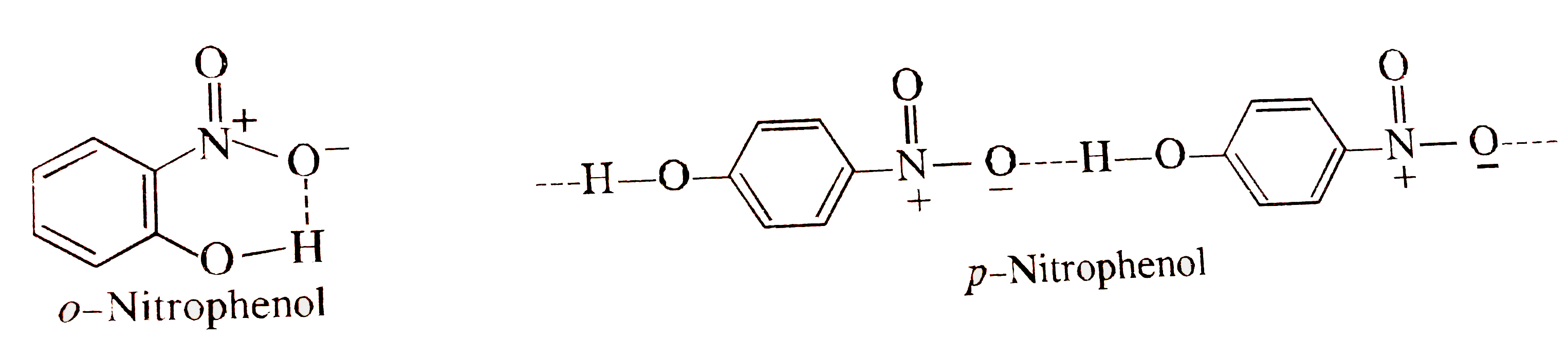Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Account for the following : Ortho nitrophenol is volatile than para ...
Text Solution
|
- Which of the following compounds are steam volatile ? (i) para-Hydroxy...
Text Solution
|
- The boiling point of para nitrophenol is greater than that of ortho ni...
Text Solution
|
- Assertion : Boiling point of p-nitrophenol is greater than that of o-n...
Text Solution
|
- The order of melting point of ortho, para, meta-nitrophenol is
Text Solution
|
- The order of melting point of ortho, para, meta nitrophenol is
Text Solution
|
- Ortho and para-nitrophenols are separated by which of the following me...
Text Solution
|
- Ortho isomer of nitrophenol is steam volatile due to .
Text Solution
|
- Which is steam volatile: ortho- or p-nitrophenol? Give reason also. or...
Text Solution
|