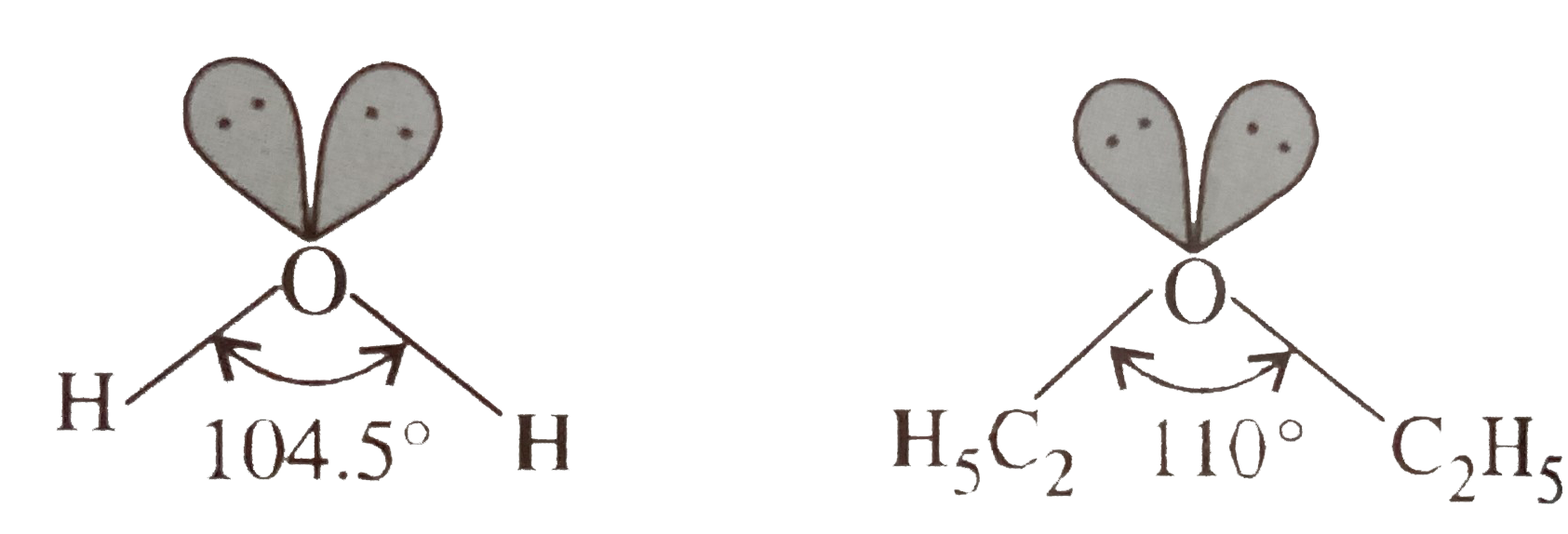Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Though ethers are the derivatives of water, the two differ in bond ang...
Text Solution
|
- The hybridisatio of oxygen in both water and diethyl ether molecules i...
Text Solution
|
- C-O-C bond angle in ether is more than H-O-H bond angle in water altho...
Text Solution
|
- (A) The C-O-C bond angle in ethers is higher than H-O-H bond angle in ...
Text Solution
|
- The four C-H bonds in methane are equivalent though carbon was differe...
Text Solution
|
- The bond angle and hybridization in ether (CH3OCH3) is :
Text Solution
|
- জল এবং ডাই ইথাইল ইথার উভয়ক্ষেত্রেই কেন্দ্রীয় O- পরমাণুর সংকরায়ন এক...
Text Solution
|
- जल और डाइएथिल ईथर अणु दोनों में ऑक्सीजन का संकरण समान होता है परन्तु य...
Text Solution
|
- Bond angle in dimethyl ether is more than that in water.
Text Solution
|