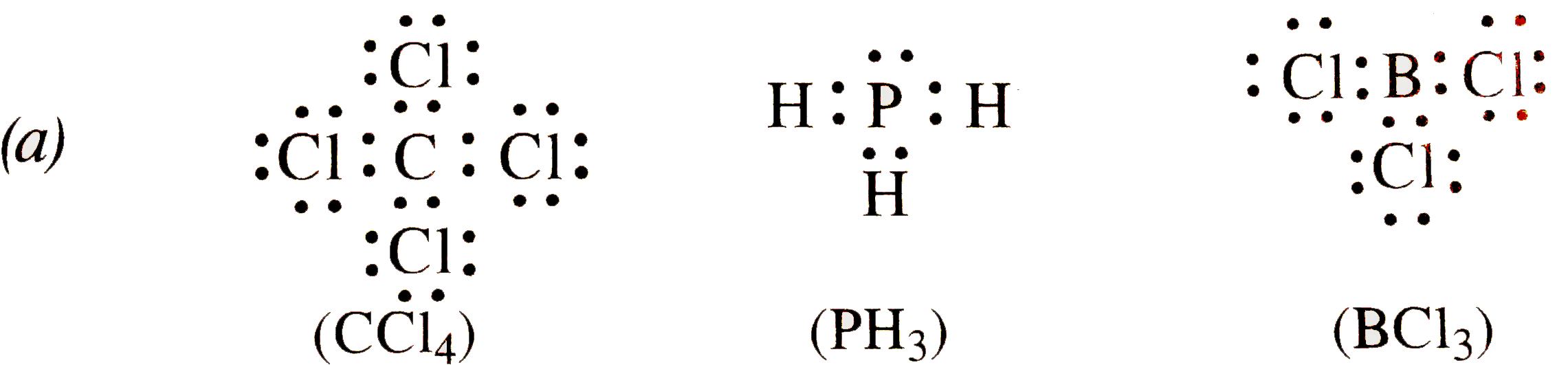Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the Lewis dot structures of (a) C Cl(4) (b) PH(3) ( c) BCl(3). I...
Text Solution
|
- Assuming a Lewis structure for SO(2) in which all the atoms obey the o...
Text Solution
|
- In the reaction BCl(3) + PH(3) rarr Cl(3)B rarr PH(3), Lewis base is
Text Solution
|
- निम्न अभिक्रिया में कौन लुइस क्षार हैं? BCl(3) + PH(3) to Cl(3)B : P...
Text Solution
|
- Draw the resonance structures of N(2)O obeying the octet rule.
Text Solution
|
- Draw Lewis dot structures for the following molecules : {:((a)C Cl(4),...
Text Solution
|
- Give the Lewis dot structures of NH(3), CH(4) and SO(3) .
Text Solution
|
- (a) C CI(4) (b) PH(3) और (c) BCI(3) की लुइस बिन्दु संरचनाएँ लिखो। क्या...
Text Solution
|
- (A): CH(3) = overset(oplus)(C )- underset(..)overset(..)(O) is more st...
Text Solution
|