Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
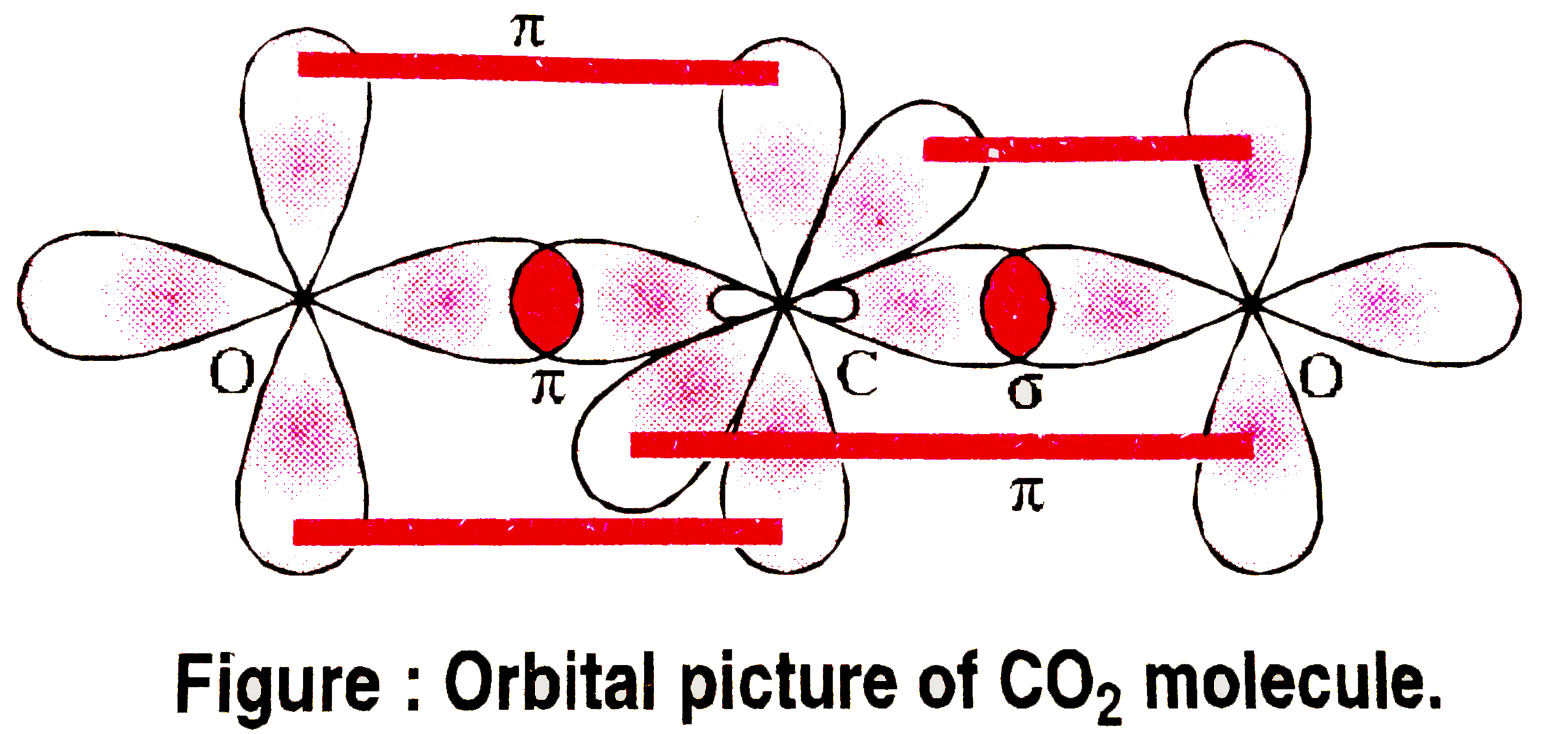
- Explain the structure of CO(2) molecule.
Text Solution
|
- Explain the structure of CO(3)^(2-) ion in terms of resonance (b) Ex...
Text Solution
|
- Explain the structure of CO(2) molecule.
Text Solution
|
- Draw the resonating structures fo CO(2) molecule.
Text Solution
|
- Explain the reasonance structure of CO(2) molecule?
Text Solution
|
- Explain the sturcture of CO(2) molecule in terms of reasonance,
Text Solution
|
- निम्नलिखित अणुओं तथा आयनों की लुईस संरचनाएँ लिखिए । CO(3)^(-2)
Text Solution
|
- Write the resonating structures for the following molecules. CO(2)
Text Solution
|
- Write the resonating structures for the following molecules. CO(3)^(...
Text Solution
|