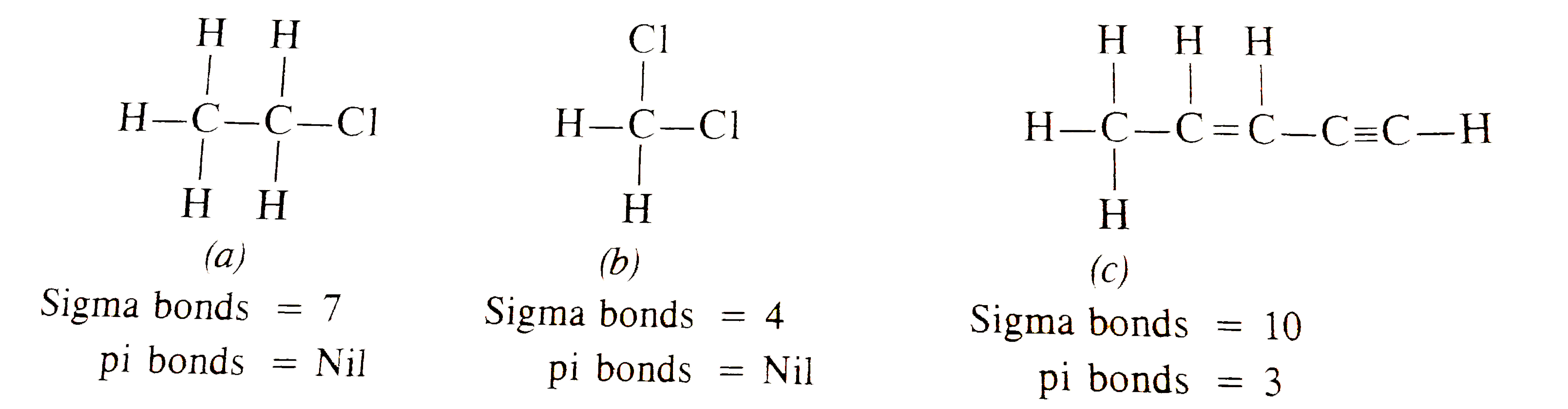Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find out the number of sigma and pi bonds in the following molecules. ...
Text Solution
|
- Given the IUPAC names of the following compounds: (i) CH(3)CH(2)CH(2)u...
Text Solution
|
- The number of sigma (sigma) and pi(pi) bonds in the following structur...
Text Solution
|
- How many sigma and pi bonds are present in each of the following molec...
Text Solution
|
- The IUPAC name of {:(CH(3)-C=C--CH-CH(2)-C-=CH),(" | ...
Text Solution
|
- Indicate the number of sigma -and pi -bonds in the following molecules...
Text Solution
|
- How many bonds (sigma and pi ) are there in the following molecules? (...
Text Solution
|
- The number of sigma bonds in CH(2)=CH - CH = CH - C -= CH is .
Text Solution
|
- Find the number of sigma-and pi-"bond" in the molecule: CH(3)CH(2)CH=C...
Text Solution
|