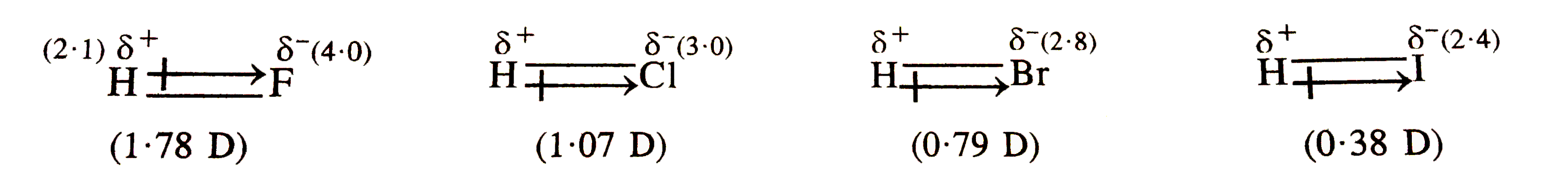Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The dipole moment of hydrogen halides decreases form HF to HI. Explain...
Text Solution
|
- Give the decreasing order of dipole moments of HF,HCI,HBr , and HI .
Text Solution
|
- The decreasing order of dipole moments of the molecules HF,H(2)O,BeF(2...
Text Solution
|
- Explain why dipole moment of hydrogen halides decreases from HF to HI
Text Solution
|
- द्विध्रुव आघूर्ण का घटता क्रम है:
Text Solution
|
- HF, HCl, HBr, HI को बढ़ते हुए द्विध्रुव आघूर्ण के क्रम में लिखिए ।
Text Solution
|
- हाइड्रोजन हैलाइडों में HF प्रबलतम अम्ल है।
Text Solution
|
- हाइड्रोजन हैलाइडों का द्विध्रुव आघूर्ण का घटता हुआ क्रम है- H-F gt H...
Text Solution
|
- Explain why the dipole moment values of hydrogen halides decrease from...
Text Solution
|