Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
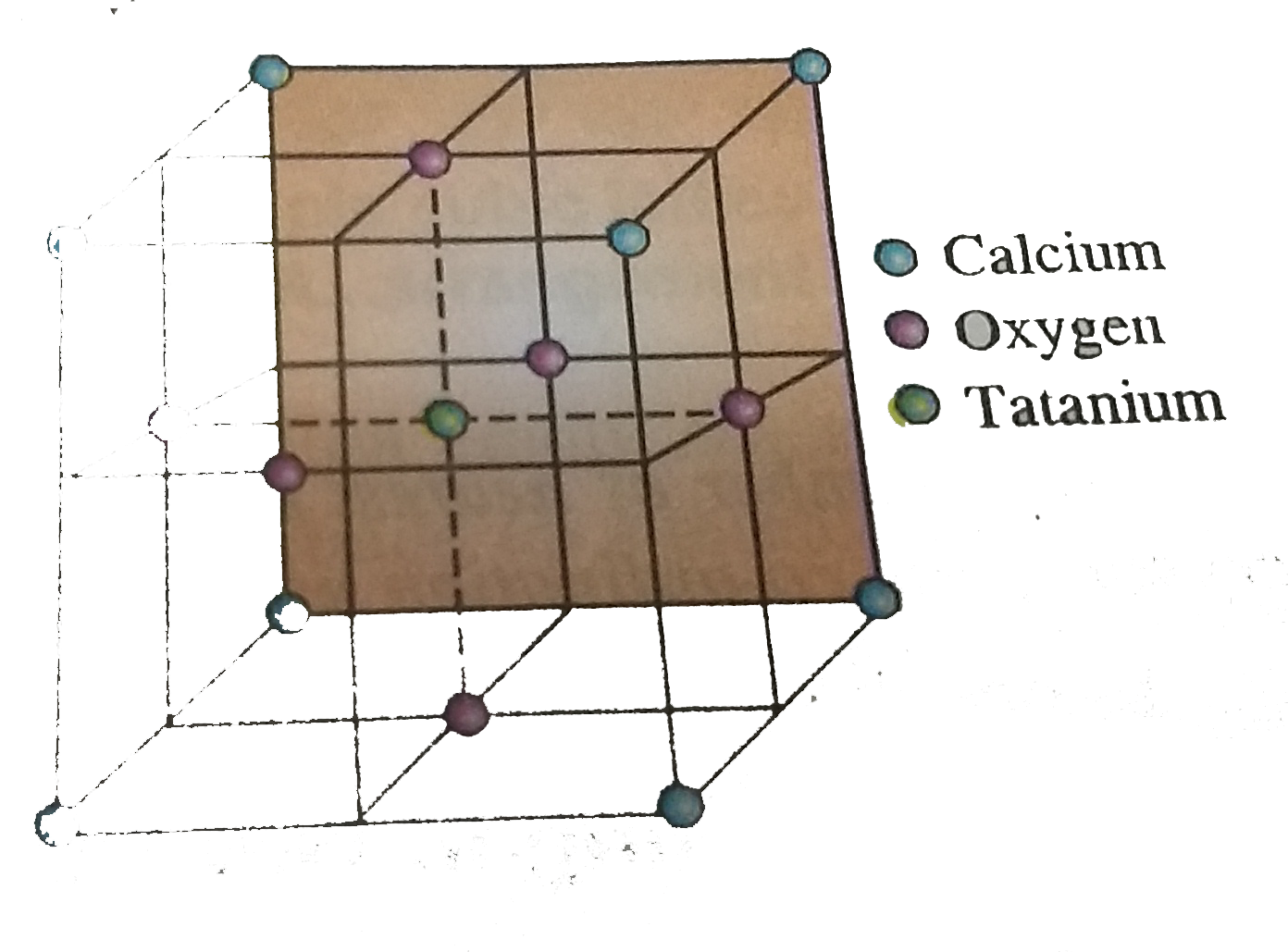
- A mineral containing calcium, oxygen and titanium crystallises in a gi...
Text Solution
|
- The mineral rutile is an oxide of titanium containing 39.35% oxygen an...
Text Solution
|
- The figure below shown a unit cell of the mineral perovskite (the tita...
Text Solution
|
- The figure below shows a unit cell of the mineral Perovskite ( the tit...
Text Solution
|
- Perovaskite, a mineral containing calcium, oxygen & titanium crystalli...
Text Solution
|
- Perovaskite, a mineral containing calcium, oxygen & titanium crystalli...
Text Solution
|
- Perovaskite, a mineral containing calcium, oxygen & titanium crystall...
Text Solution
|
- Perovskite is mineral containing calcium, oxygen and titanium, in whi...
Text Solution
|
- A mineral is made of calcium ,titanium and oxygen Ca^(2+) ions located...
Text Solution
|