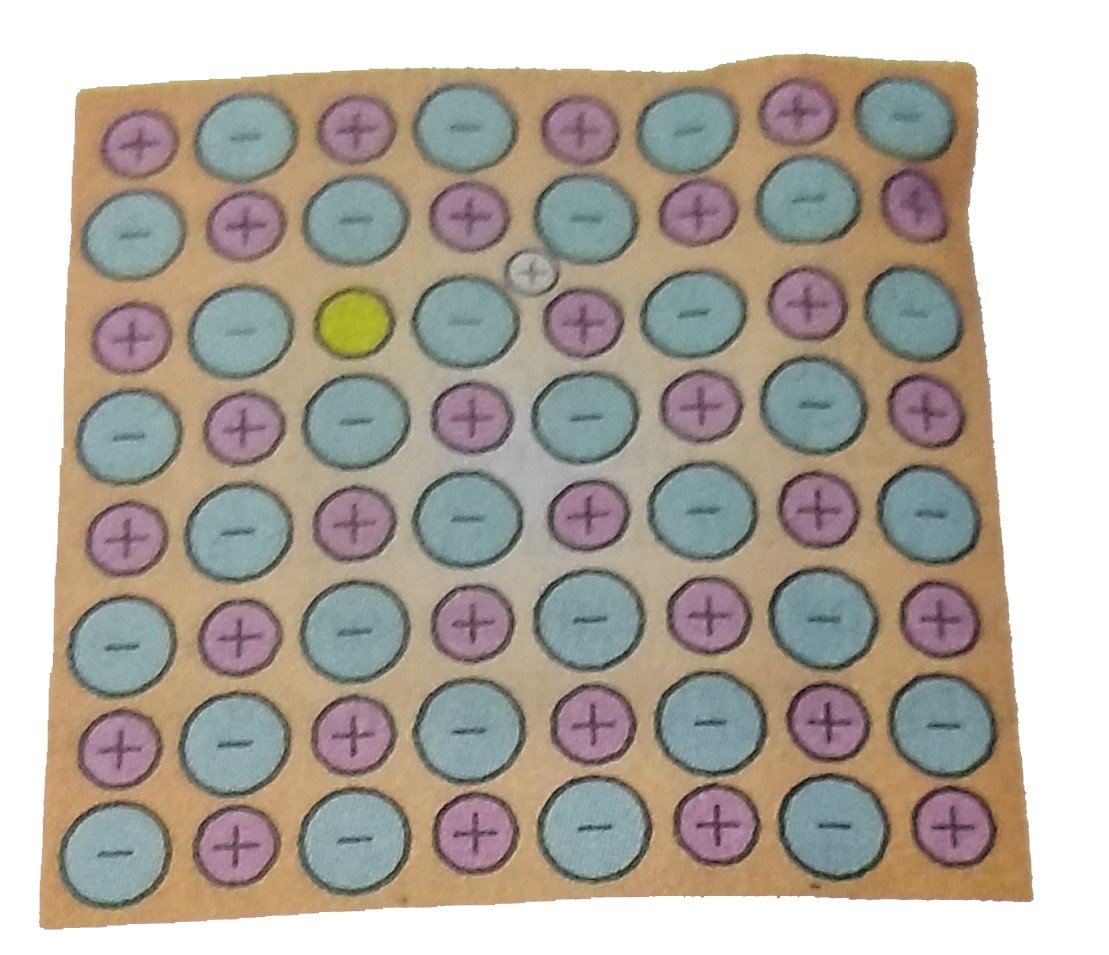
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the figure of a defective crystal : (a) Name the defect sho...
Text Solution
|
- Examine the illustration of a portion of the deffective crystal and an...
Text Solution
|
- Explain Frenkel defect in ionic crystals. What type of compounds exhib...
Text Solution
|
- Examiine the given defective crystal (i) What type of stoichiometric d...
Text Solution
|
- What are stoichiometric defects or intrinsic defects in ionic crystals...
Text Solution
|
- What is Schottky defect? Is it a stoichiometric or non-stoichiometric ...
Text Solution
|
- What is Frenkel defect? What type of ionic solids are likely to develo...
Text Solution
|
- Name the ionic solid which shows both Schottky defect and Frenkel defe...
Text Solution
|
- Defects in ionic solid|Types of stoichiometric defects|Schottky defect
Text Solution
|