A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
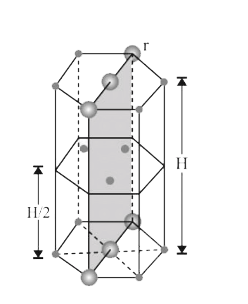
- In hexagonal systems of crystals, a frequently encountered arrangement...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In hexagonal system of crystals, a frequently encountered arrangement ...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In hexogonal systems of crystals, a frequently encountered arrangement...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In hexogonal systems of crystals, a frequently encountered arrangement...
Text Solution
|