Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
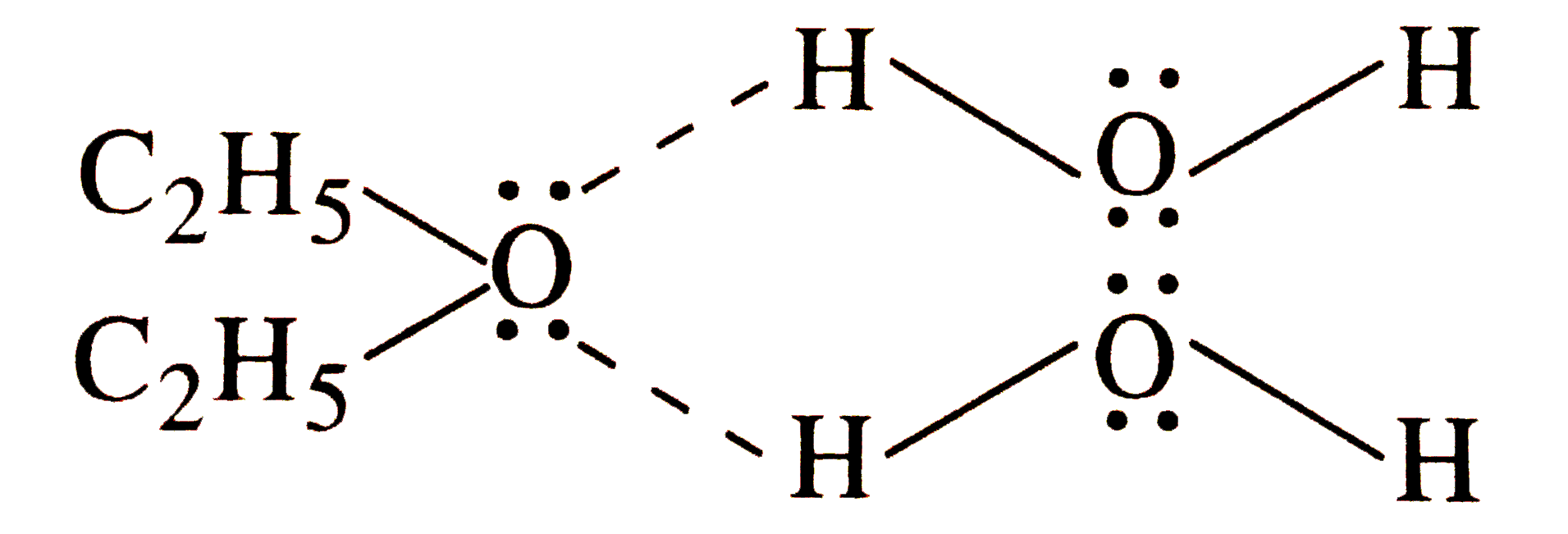
- How do you account for the miscibility of ethoxyethane with water?
Text Solution
|
- (a) What holds the atmosphere around earth? (b) How do you account for...
Text Solution
|
- How do you account for the function of mud guards ?
Text Solution
|
- How will you separate a solution (miscible) of benzene and chloroform?
Text Solution
|
- How is it that alcohol and water are miscible inall proportions ?
Text Solution
|
- How will you account for 104.5^(@) bond angle in water ?
Text Solution
|
- How do you account for the inert character of dinitrogen ?
Text Solution
|
- How is it that alcohol and water are miscible inall proportions ?
Text Solution
|
- how is that alcohol and water are miscible in all proportions?
Text Solution
|