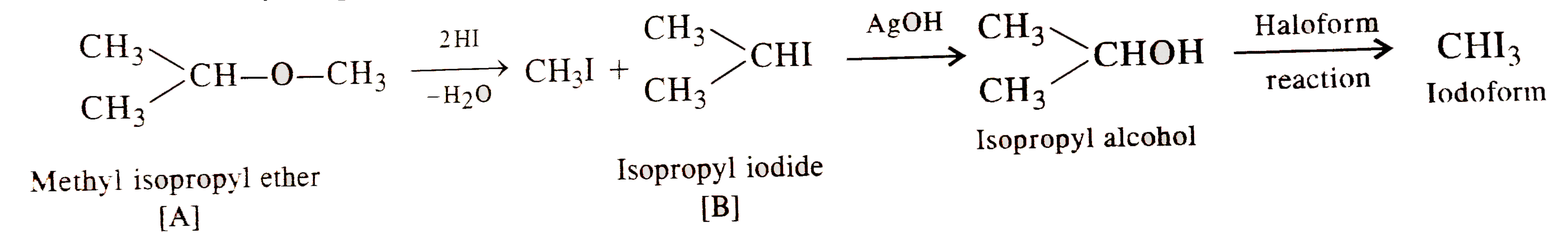Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A neutral compound (A) having C,H and O, on refluxing with HI yields m...
Text Solution
|
- When ethyl bromide is treated with moist Ag(2)O main product is//are.
Text Solution
|
- When alkyl halide is heated with dry Ag(2)O . It produces :
Text Solution
|
- Aqueous ZnSO(4) treated with Na(2)CO(3)//H(2)O produces A which when h...
Text Solution
|
- Two compounds [A] and [B] have molecular formula C(2)H(6)O. On reactin...
Text Solution
|
- Silver iodide is used for producing artificial rain because Ag(2)I
Text Solution
|
- Ethyl iodide reacts with most Ag(2)O to form
Text Solution
|
- An ether, (A) having molecular formula, C(6)H(14)O, when treated with ...
Text Solution
|
- शुष्क Ag(2)O के साथ क्रिया करके एल्किल हैलाइड देते है:
Text Solution
|