A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
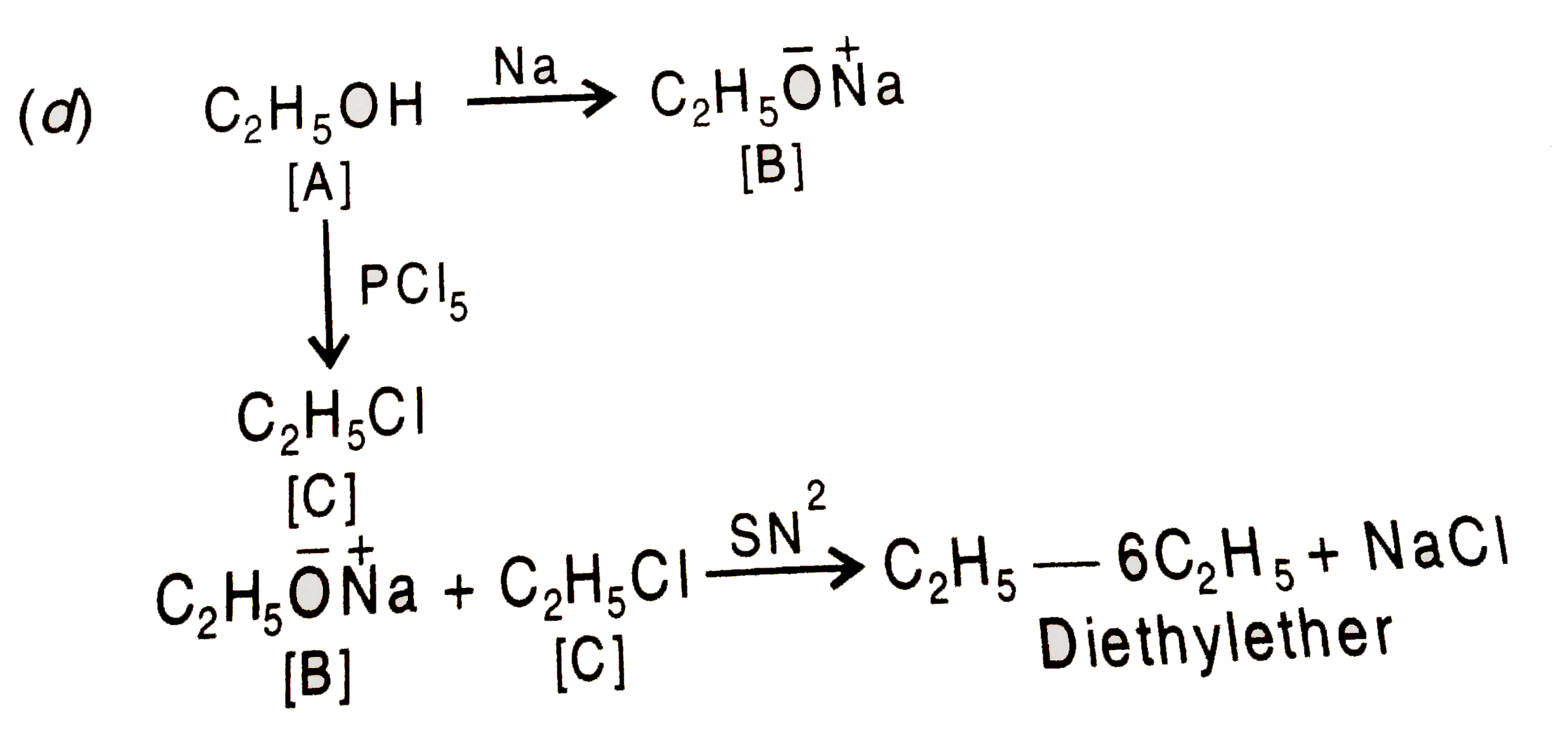
- The compound A on treatment with Na gives B, and with PCl(5) gives C. ...
Text Solution
|
- The compound A on treatement with Na gives B , and with PCI(5) gives C...
Text Solution
|
- Compound A reacts with Na metal to give B. A also reacts with PCl(5) t...
Text Solution
|
- An alkali metal A gives a compound B (molecular mass = 40) on reacting...
Text Solution
|
- An alkali metal 'A' gives a compound B (molecular mass = 56) on reacti...
Text Solution
|
- A nitraite on acid hydrolysis gives compound A, which reacts with thio...
Text Solution
|
- यौगिक A सोडियम से क्रिया कर B देता है एवं PCl(5) से क्रिया कर स देता ह...
Text Solution
|
- An organic compound A forms B with Na metal. A also forms C with PCl...
Text Solution
|
- The compound (A) on treatment with Na gives (B) and with SOCl(2) gives...
Text Solution
|