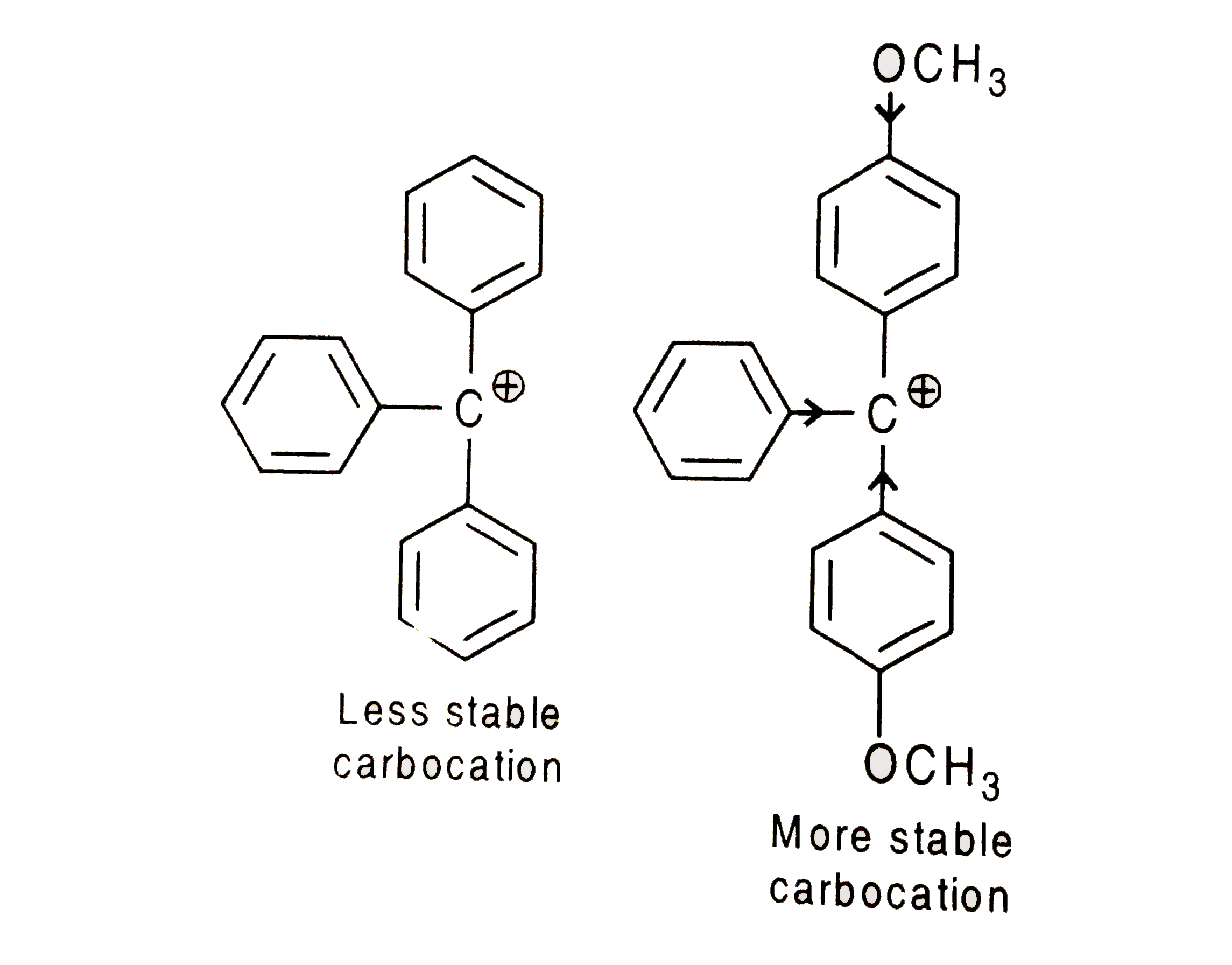A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
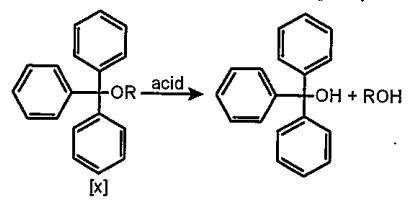
- The acidic hydrolysis of ether (X)shown below is fastest when
Text Solution
|
- The acidic hydrolysis of ether (X) shown below is fastest when
Text Solution
|
- The acidic hydrolysis of ether (X) shown below is fastest when
Text Solution
|
- The total number of distinct naturally occuring amino acids obtained b...
Text Solution
|
- The acidic hydroysis of ether (X)shown below is fastest when :
Text Solution
|
- Hydrolysis of which of the following gives acid at fastest rate :
Text Solution
|
- Oxygen when passed through an ethereal solution of phenyl magnesium io...
Text Solution
|
- The reaction is fastest when X is
Text Solution
|
- Enlist the total number of distinct naturally occurring amino acids ob...
Text Solution
|