A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion : Ethers have specific dipole moment values Reason : The C...
Text Solution
|
- POLARITY OF BONDS- DIPOLE MOMENT
Text Solution
|
- All molecules with polar bonds have dipole moment.
Text Solution
|
- Which of the following molecules will have polar bonds but zero dipole...
Text Solution
|
- ध्रुवीय आबंधो युक्त सभी अणुओं के द्विध्रुव आघूर्ण का एक निश्चित ...
Text Solution
|
- Which of the following molecules will have polar bonds but zero dipole...
Text Solution
|
- Assertion : All diatomic molecules with polar bond have dipole moment....
Text Solution
|
- ध्रुवीय बन्ध (polar bond) वाले सभी अणु द्विध्रुव आघूर्ण (dipole moment...
Text Solution
|
- Which of the following molecules will have polar bonds but zero dipole...
Text Solution
|
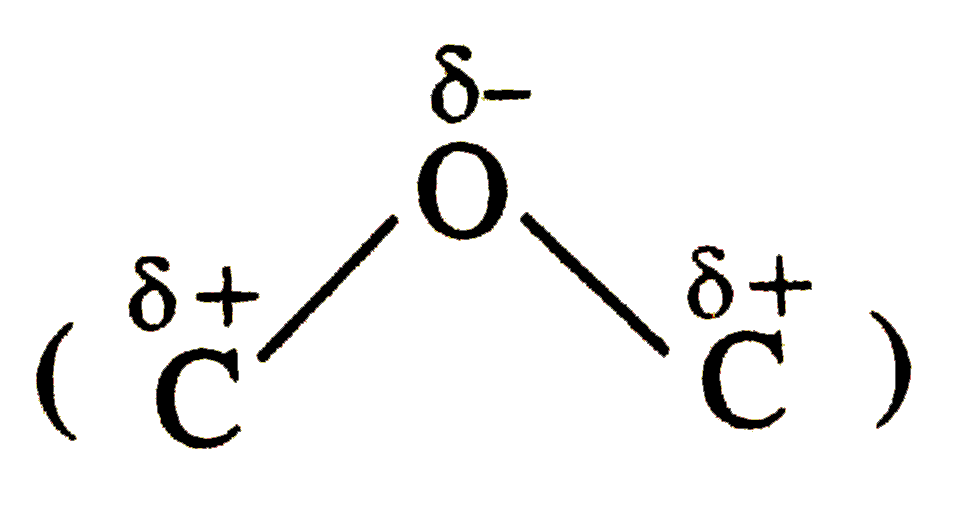 bond do not cancel out.
bond do not cancel out.