Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 0.44 g of a monohydric alcohol when added to methylmagnesium iodide in...
Text Solution
|
- 0.44g of a monohydric alcohol when added to CH(3)MgI in ether librates...
Text Solution
|
- What are monohydric alcohols ? How are they classified ?
Text Solution
|
- 0.44 g of a monhydric alcohol when added to methyl magnesium iodide in...
Text Solution
|
- Monohydric alcohols are .
Text Solution
|
- Monohydric alcohols are functional isomers of .
Text Solution
|
- ethers are different form its corresponding isomeric monohydric alcoho...
Text Solution
|
- 0.44 g of a monohydric alcohol when added to methyl magnesium iodide i...
Text Solution
|
- 0.44 g of a monohydric alcohol when added to methyl magnesium iodide i...
Text Solution
|
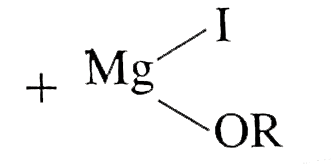 ,(0.44g,11cm^(3),):}`
,(0.44g,11cm^(3),):}` 