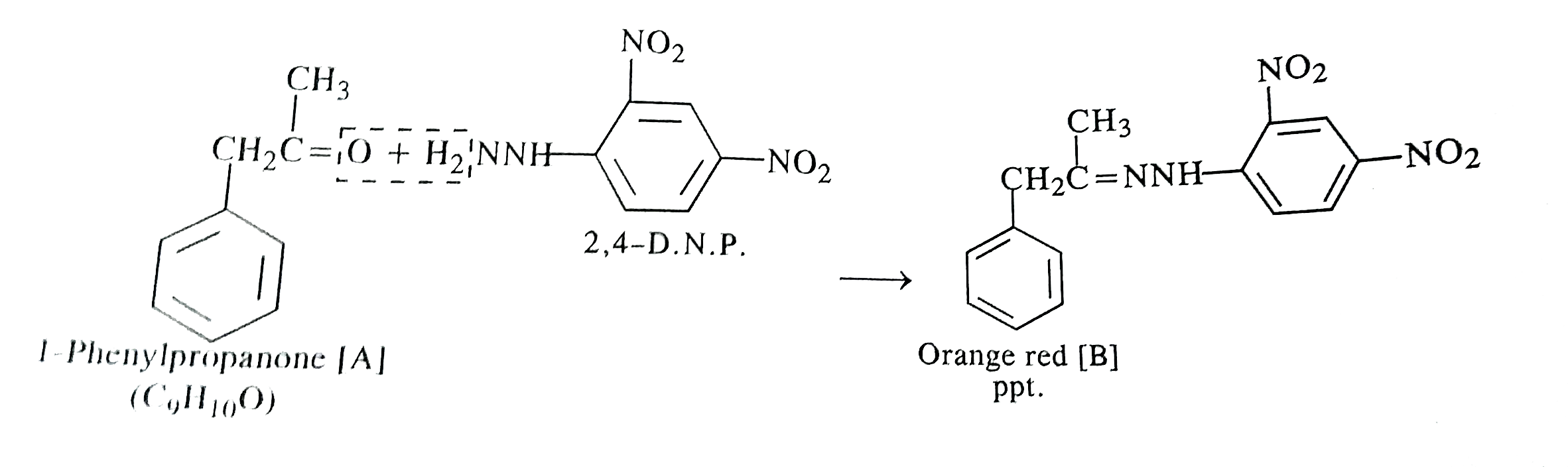
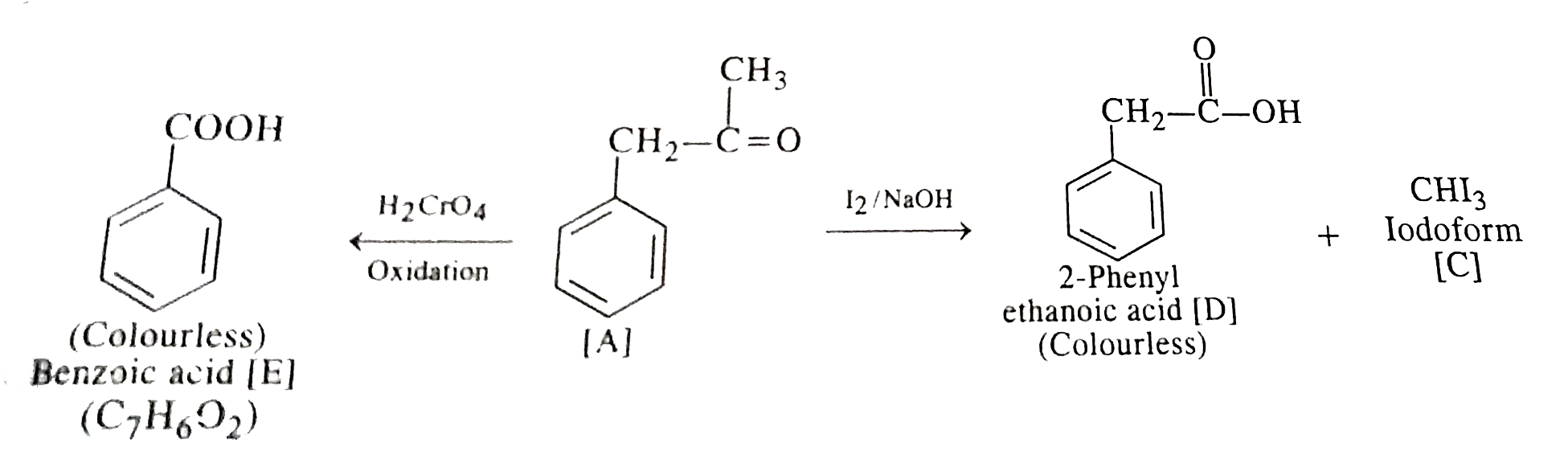
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound (A) with molecular formula C(8)H(8)O forms an oran...
Text Solution
|
- An organic compound (A) with molecular formula C8H8 O forms an o...
Text Solution
|
- An organic compound 'P' with jmolecular formula C(8)H(8)O forms an org...
Text Solution
|
- An organic compound [A] with molecular formula C(9)H(10)O forms an ara...
Text Solution
|
- An organic compound (A) whose molecular formula C(6)H(8)O Is, 2, -4 pr...
Text Solution
|
- एक कार्बनिक यौगिक (A) जिसका आण्विक सूत्र C8H8 O है , डाईट्रोफेनील हाइ...
Text Solution
|
- An organic compound (A) with molecular formula C(8)H(8)O forms an oran...
Text Solution
|
- An organic compound (A) with molecular formula C8H8 O forms an o...
Text Solution
|
- An organic compound (A) with molecular formula C(8)H(8)O forms an oran...
Text Solution
|