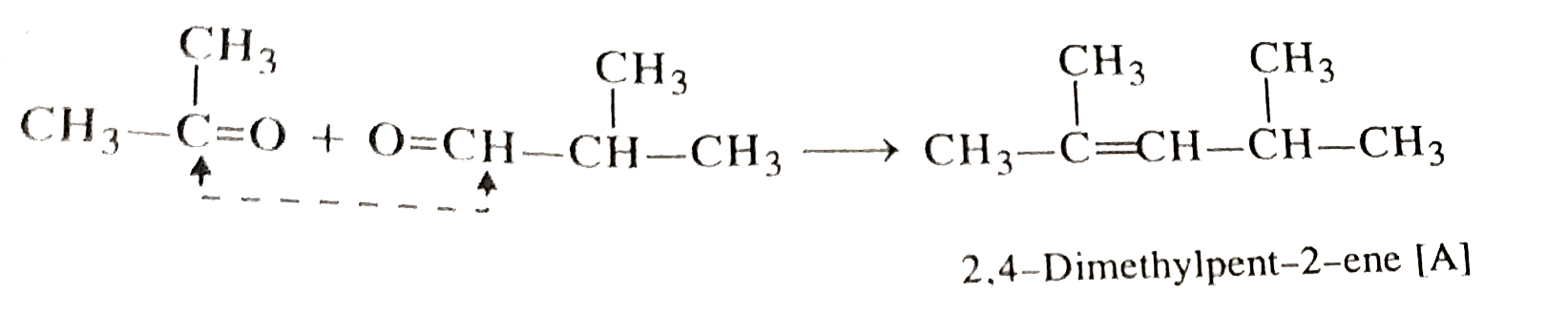Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
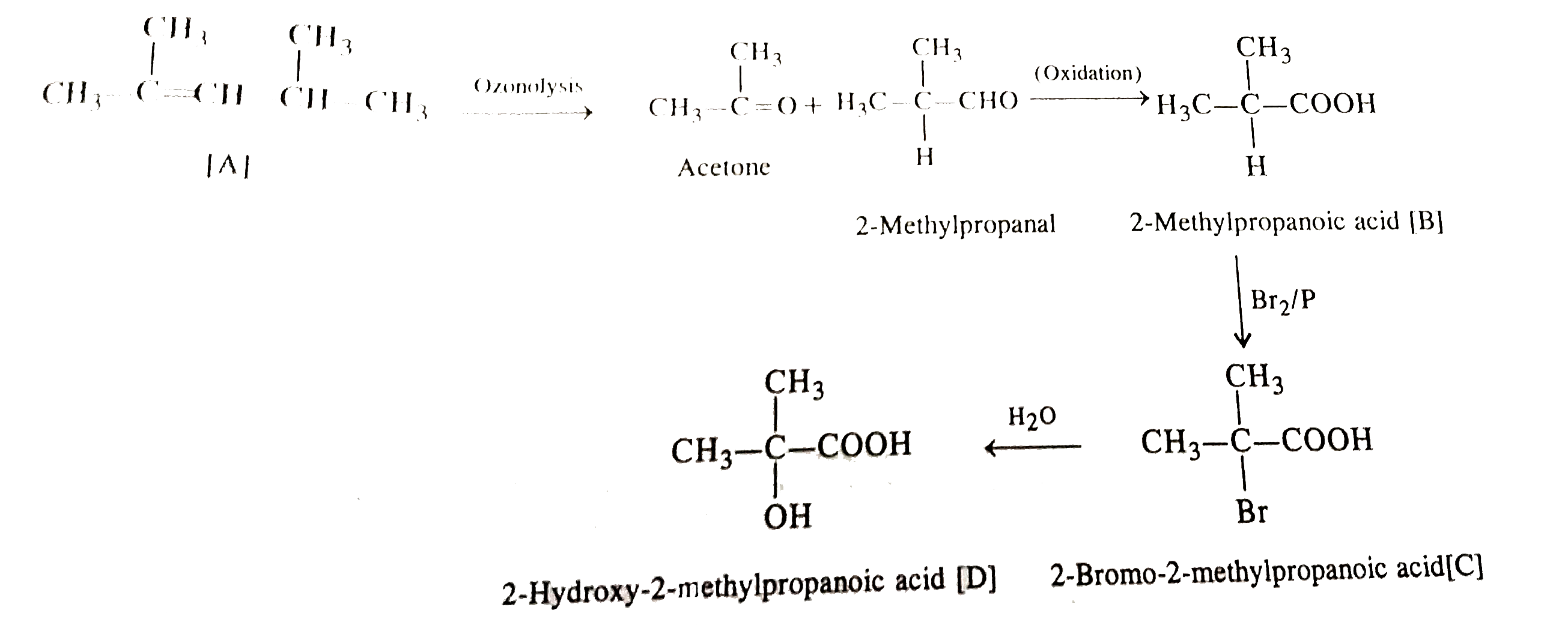
- An alkane A on ozobolysis yields acetone and an aldeyde. The aldehyde ...
Text Solution
|
- Which of the following compounds on hydrolysis yields acetic acid?
Text Solution
|
- A compound (A) of boron reacts with Nme(3) to give an adduct (B) which...
Text Solution
|
- Acid hydrolysis of which of the following compounds yields two differe...
Text Solution
|
- Acid hydrolysis of which of the following compounds yields two differe...
Text Solution
|
- A compound (A) of boron reacts with NMe(3) to give an adduct (B) which...
Text Solution
|
- Which of the following compounds on hydrolysis yields a carboxylic aci...
Text Solution
|
- A nitraite on acid hydrolysis gives compound A, which reacts with thio...
Text Solution
|
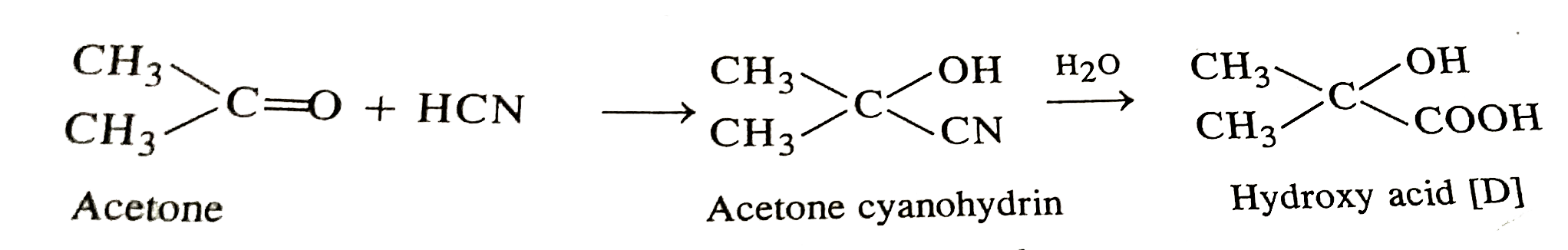
- A carbonyl compound 'A'' react with hydrogen cyanide to give cyanohydr...
Text Solution
|