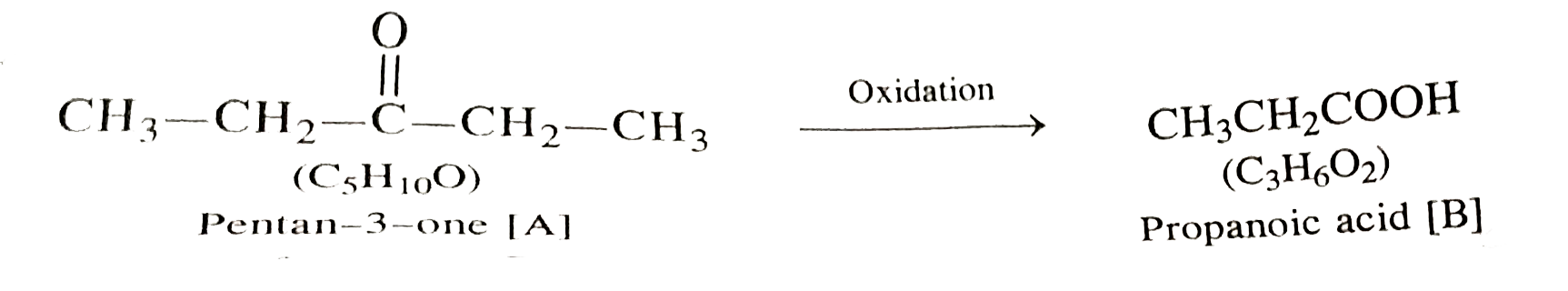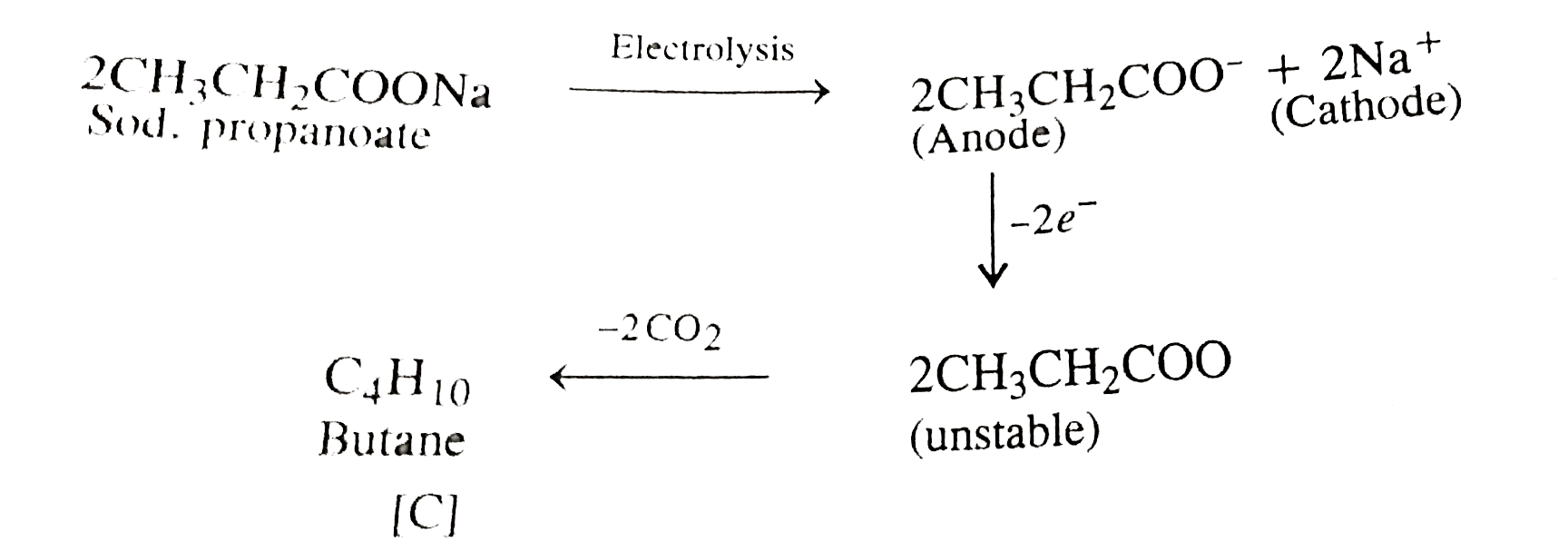Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A compound [A] with molecular formula C(5)H(10)O gave a positive 2,4-D...
Text Solution
|
- A compound 'A' formula of C(3)H(6)CI(2) on reaction with alkali can gi...
Text Solution
|
- An aromatic compound 'A' (Molecular formula C(8)H(8)O) ) gives positiv...
Text Solution
|
- A compound 'A' having the molecualr formula C(5)H(12)O on oxidation gi...
Text Solution
|
- Hydrocarbon [A] with molecular formula C(8)H(8) gave the following rea...
Text Solution
|
- Compound (A) C(6)H(12) gives a positive test with bromine in carbon te...
Text Solution
|
- An organic compound 'A' is a constituent of antifreeze and has the mol...
Text Solution
|
- A compound 'A' having the molecular formula C(5)H(12)O, on oxidation ...
Text Solution
|
- An aromatic compound 'A' (molecular formula C(8)H(8)O ) gives positive...
Text Solution
|