Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
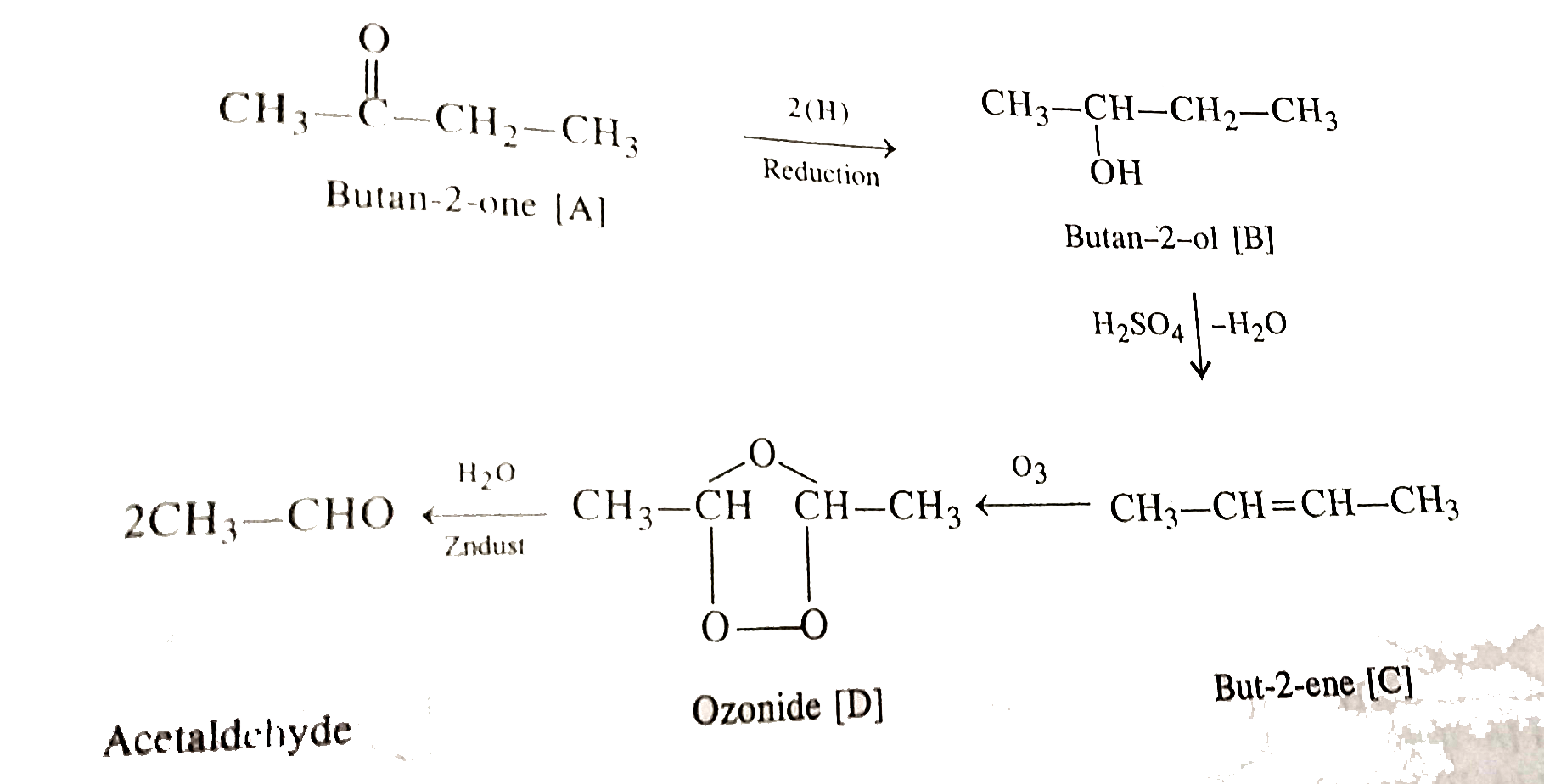
- A ketone A, which undergoes halform reaction, gives compound B on redu...
Text Solution
|
- Ketone (A), which indergoes halform reaction, give compound (B) on red...
Text Solution
|
- An element A reacts with water to form a compound B which is used in w...
Text Solution
|
- A compound (A) of boron reacts with NMe(3) to give an adduct (B) which...
Text Solution
|
- A ketone (A) which undergoes reduction to give (B). (B) on heating wit...
Text Solution
|
- A ketone A, which undergoes halform reaction, gives compound B on redu...
Text Solution
|
- An element A occupies group number 15 and period number 3, reacts with...
Text Solution
|
- An organic compound 'A' on reduction gives compound 'B' which on react...
Text Solution
|
- एक कीटोन 'A' जो हैलोफोर्म अभिक्रिया देता है, अपचयन पर यौगिक 'B' बनाता ...
Text Solution
|