Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
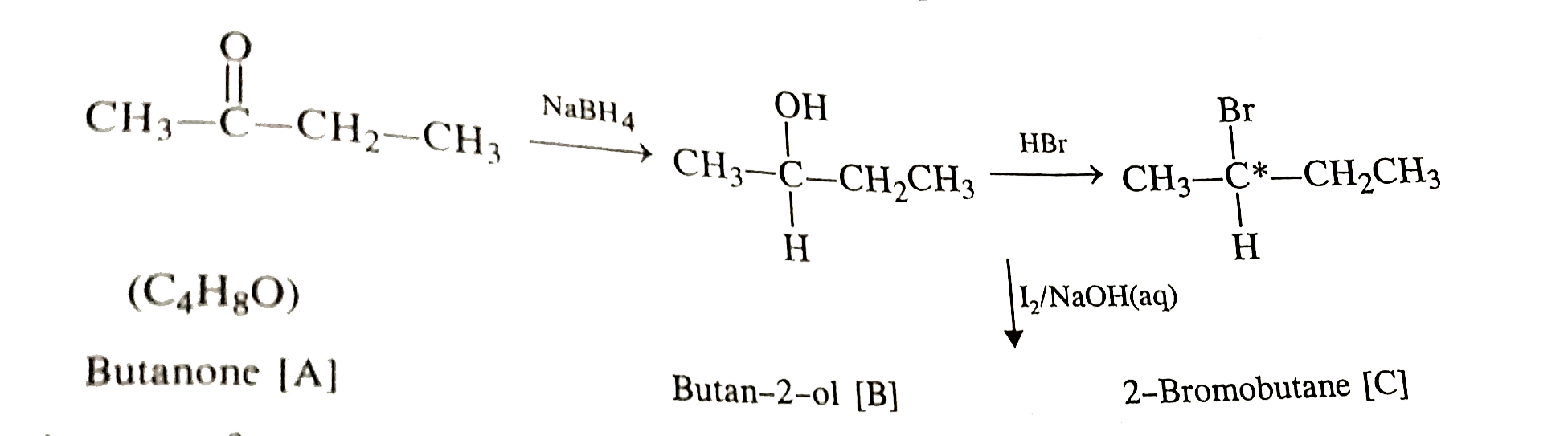
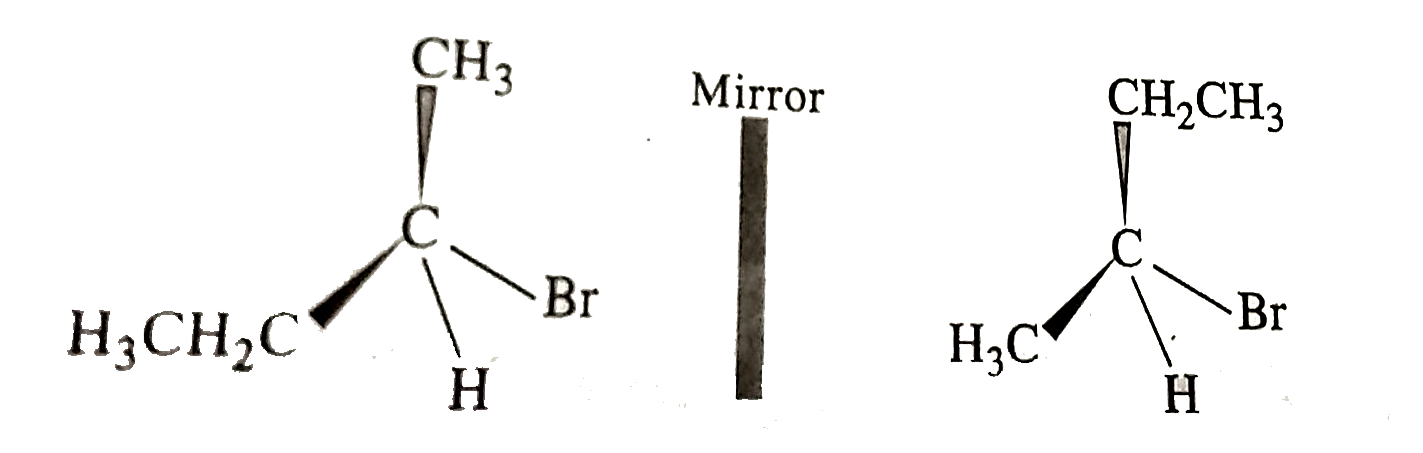
- An organic compound [A] with molecular formula C(4)H(8)O when reduced ...
Text Solution
|
- Primary alkyl halide C(4)H(9)Br (A) is reacted with alcoholic KOH to g...
Text Solution
|
- A compound (A) with molecular formula C(4)H(10)O reacts rapidly with m...
Text Solution
|
- A neutral organic compound A of molecular formula C(2)H(6)O on heating...
Text Solution
|
- An organic compound (A) with molecular formula C(2)H(5)Cl reacts with ...
Text Solution
|
- A hydrocarbon C(3)H(6)(A) reacts with HBr to form compound (B). Compou...
Text Solution
|
- Two isomers (A) and (B) have the same molecular formula C(2)H(4)Cl(2) ...
Text Solution
|
- An organic compound (A) of molecular formula C(7)H(6)O is not reduced ...
Text Solution
|
- Compound (A) of molecular formula C(7)H(8) when treated with air in pr...
Text Solution
|