Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
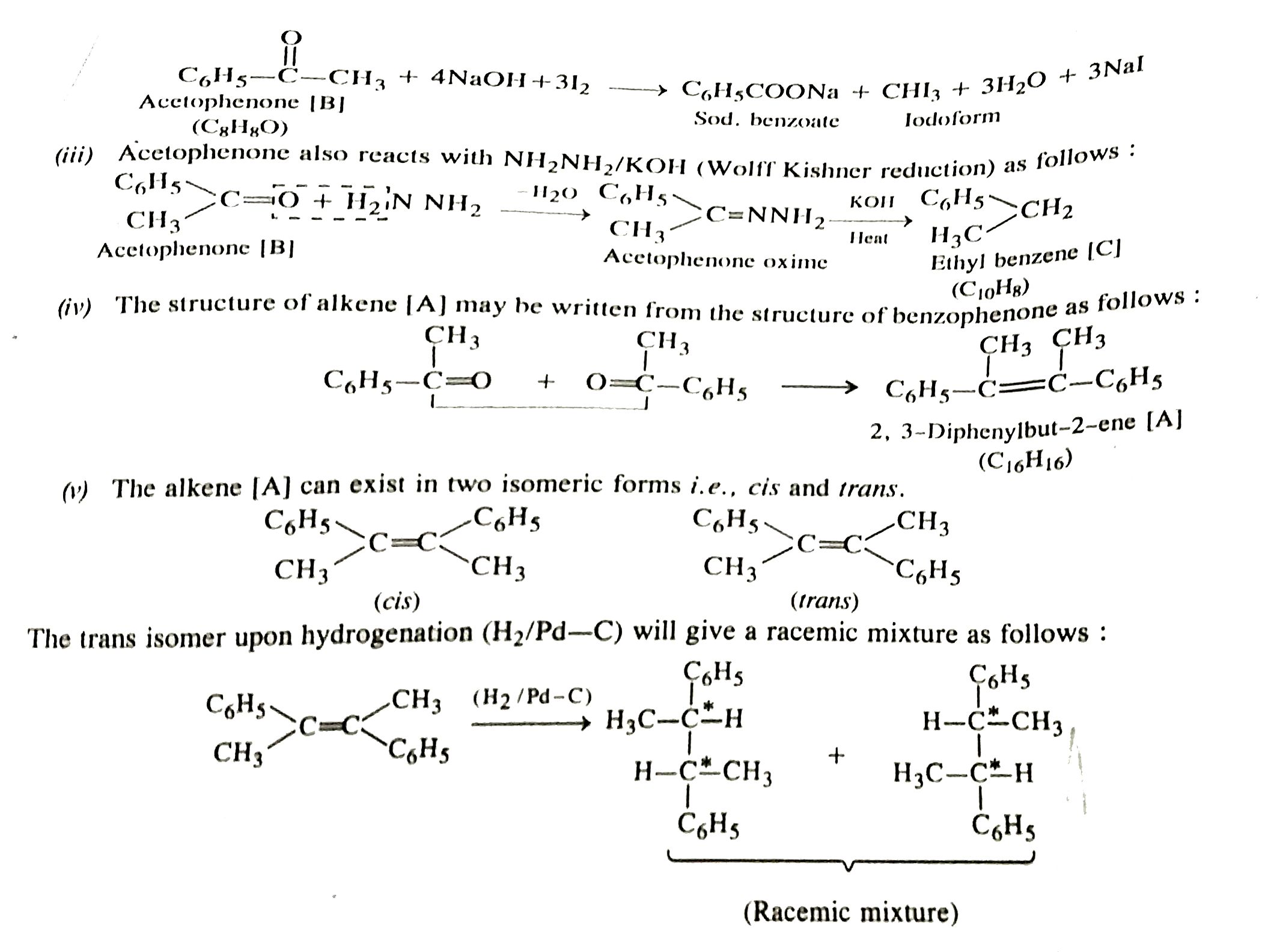
- An alkane (A) C(16)H(16) on ozonolysisi gives only one products (B) C(...
Text Solution
|
- An alkene (A) C(16)H(16) on ozonolysis gives only one product (B) (C(8...
Text Solution
|
- Primary alkyl halide C(4)H(9)Br (A) is reacted with alcoholic KOH to g...
Text Solution
|
- On ozonolysis, an organic compound (A) C(6)H(10) gives two aldehyde (B...
Text Solution
|
- Compound (A) C(5)H(8)O(2) liberated CO(2) on reaction with sodium bica...
Text Solution
|
- An alkene (A) C(16)H(16) on ozonolysis gives only one product (B) C(8)...
Text Solution
|
- Compound C(4)H(8)CI(2) (A) on hydrolysis gives a compound C(4)H(8)O(B)...
Text Solution
|
- An alkene (A) C(10)H(10) on ozonolysis gives only one product (B) C(8)...
Text Solution
|
- An alkene (A) C(16)H(16) on ozonolysis gives only one product (B) C8H8...
Text Solution
|