Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
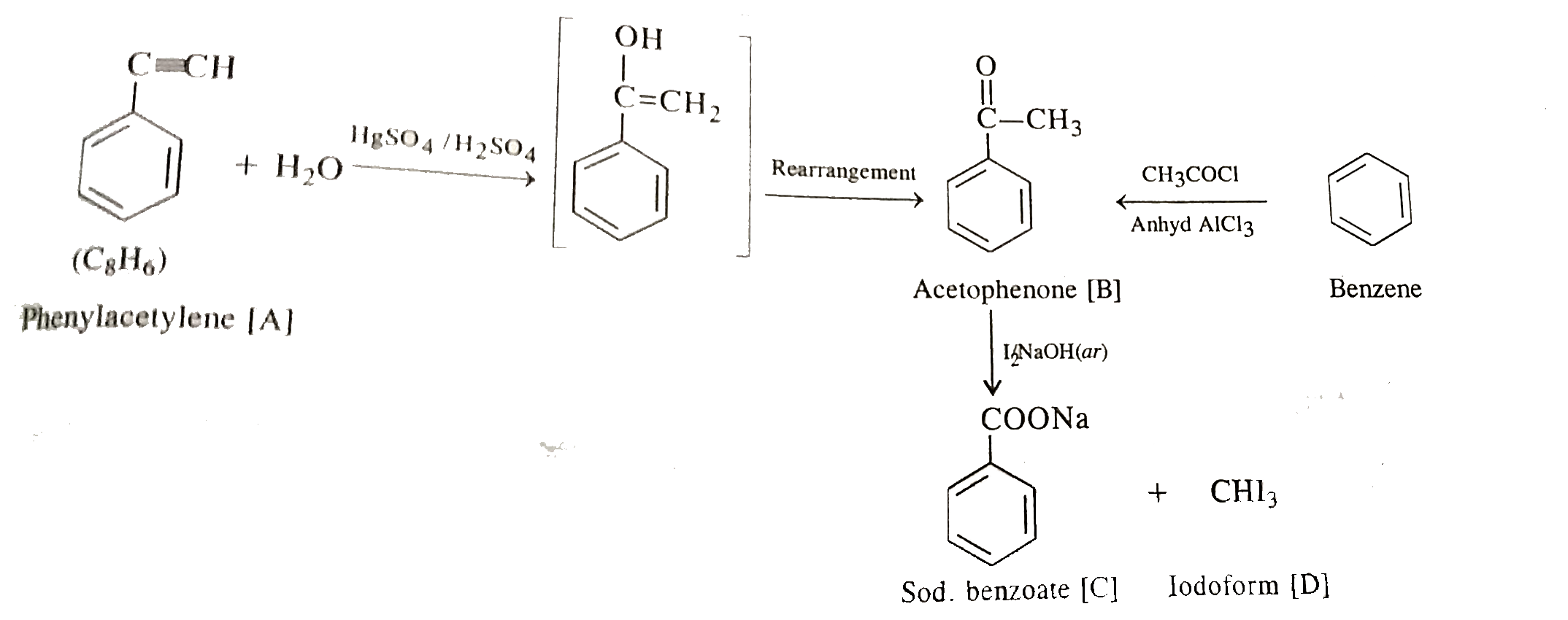
- An organic compound [A] C(8)H(6) on reacting with dilute sulphuric aci...
Text Solution
|
- An alkane A on ozobolysis yields acetone and an aldeyde. The aldehyde ...
Text Solution
|
- An organic compound [A], whose molecular formula is C(3)H(6)O, gives i...
Text Solution
|
- An organic compound A, C(8)H(6) on reacting with dil. H(2)SO(4) and Hg...
Text Solution
|
- A nitraite on acid hydrolysis gives compound A, which reacts with thio...
Text Solution
|
- n-Propyl bromide (A) when boiled with aqueous KOH undergoes hydrolysis...
Text Solution
|
- A transition metal A has 'spin-only' magnetic moment value of 1.8 Bm. ...
Text Solution
|
- When chromite ore FeCr(2)O(4) is fused with NaOH in presence of air, a...
Text Solution
|
- An organic compound (A) C(8)H(4)O(3) in dry benzene in the presence of...
Text Solution
|