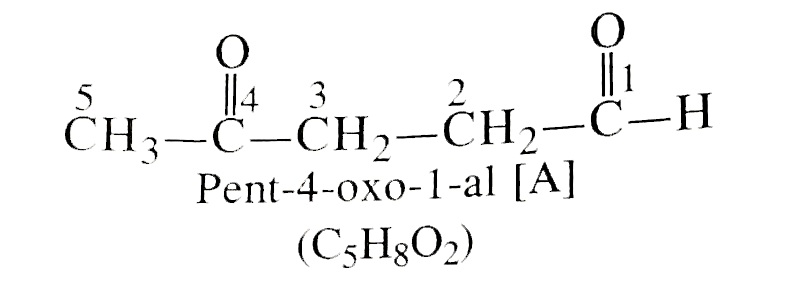Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
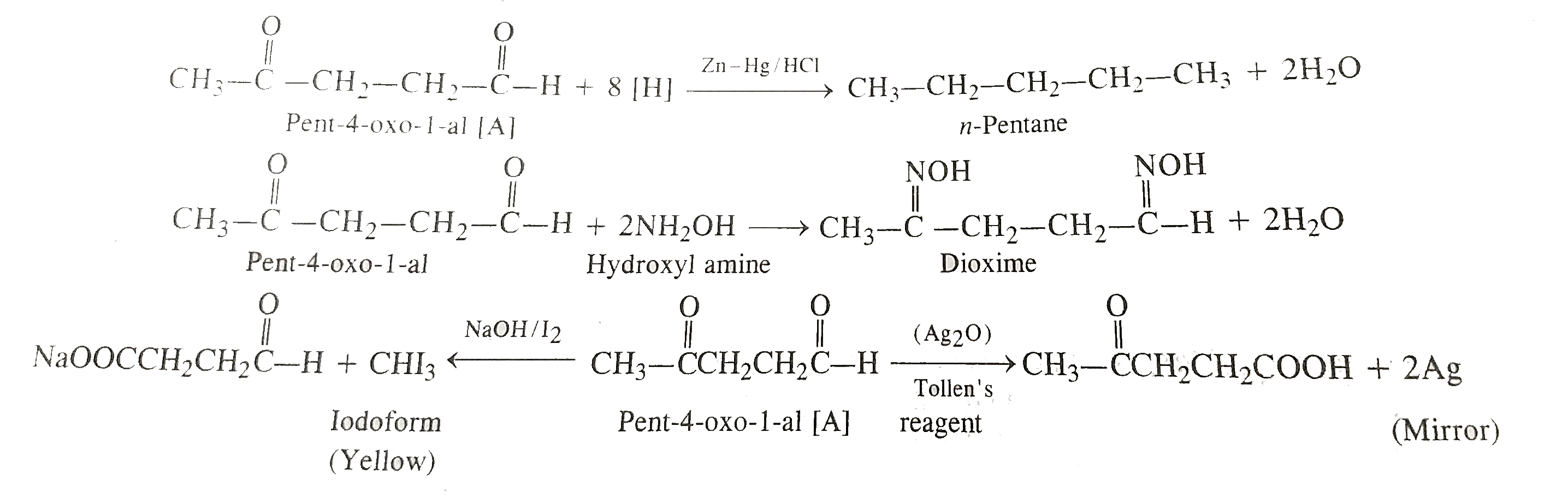
- An organic compound [A] with molecular formula C(5)H(8)O(2) is reduced...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive test with NaOH ...
Text Solution
|
- Compound 'A' C(5)H(10)O forms a phenyl hydrazone and gives a negative ...
Text Solution
|
- Draw the structure of compound (A) having molecular formula C(5)H(10)O...
Text Solution
|
- Compound (A), C(5)H(10)O, forms a phenylhdrazone, gives regative Tolle...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive test with NaOH and...
Text Solution
|
- Compound A(C(5)H(10)O) forms a phenyl hydrazon and gives a negative To...
Text Solution
|
- कोई योगिक (A) C(5)H(10)O फेनिल हाइड्राजोन बनाता है, टॉलन तथा आयोडोफॉर्...
Text Solution
|
- An organic compound of formula, C(3)H(6)O forms phenyl hydrazone, but ...
Text Solution
|