Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
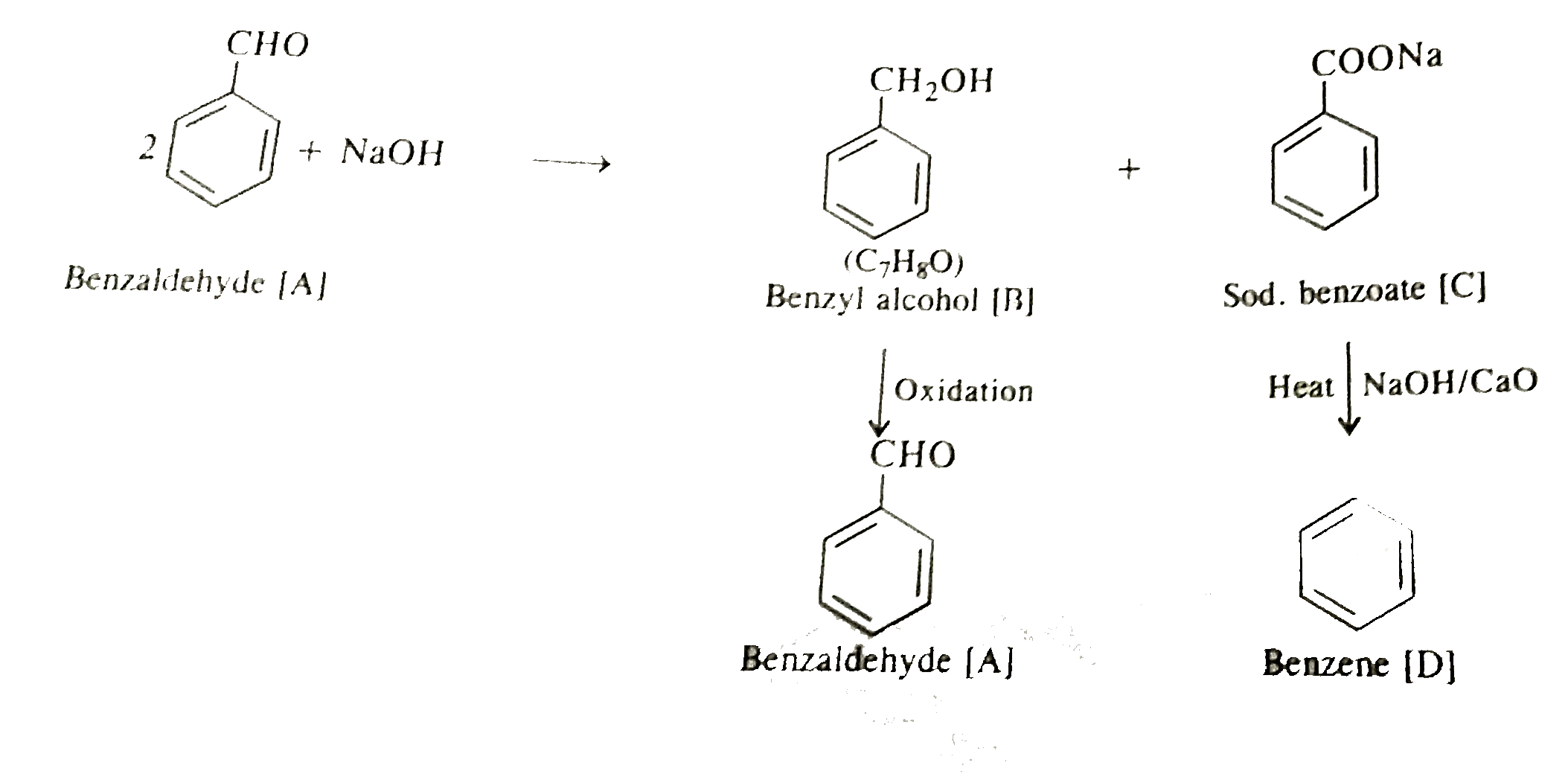
- An organic compoud [A] which has characteristic odour on treatment wit...
Text Solution
|
- An organic compound A (C(7)H(6)Cl(2)) on treatment with NaOH solution ...
Text Solution
|
- कोई कार्बनिक यौगिक (A) जिसकी लाक्षणिक गन्ध है, NaOH के साथ किया कर दो ...
Text Solution
|
- C(5)H(8)O(3) अणुसूत्र वाला एक कार्बनिक यौगिक (A) सोडालाइम के साथ गर्म ...
Text Solution
|
- एक कार्बनिक यौगिक A कॉस्टिक सोडा के सान्द्र विलयन के साथ गर्म करने पर ...
Text Solution
|
- An organic compound A reacts with sodium hydroxide to form a compound ...
Text Solution
|
- Compound A of molecular formula C(2)H(8) is treated with chlorine and ...
Text Solution
|
- An organic compound of molecula formula C(6)H(5)O"Na" is heated with C...
Text Solution
|
- A compound A of molecular formula C(3)H(7)O(2)N on reaction with Fe an...
Text Solution
|