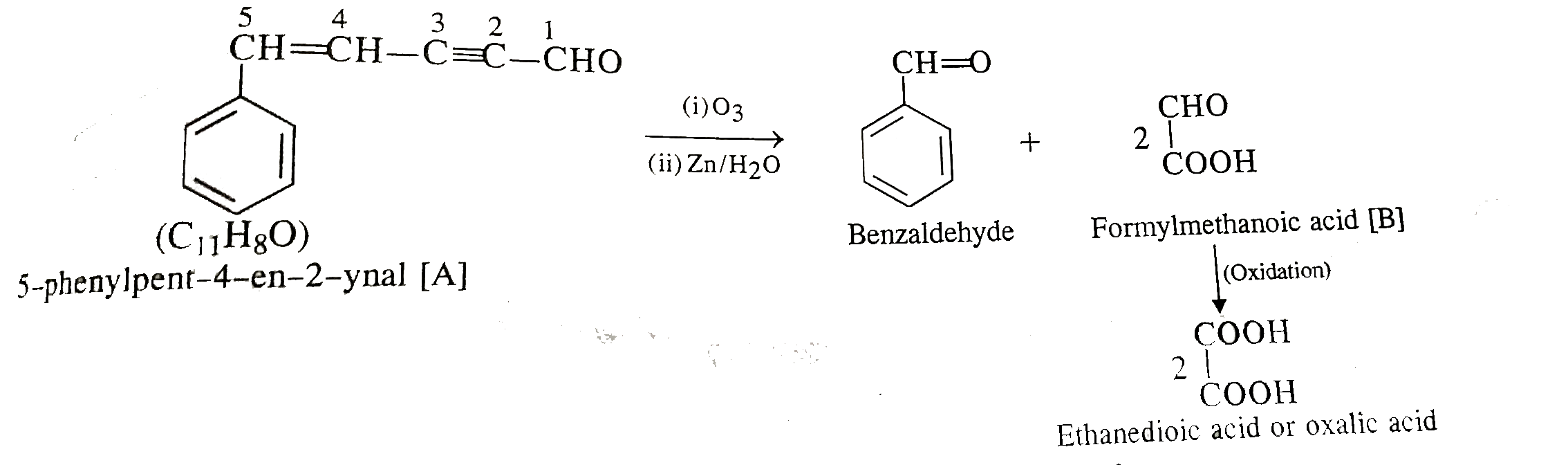Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An aldehyde (A) (C(11)H(8)O), which does not undergo self aldol conden...
Text Solution
|
- Compound (A) and (B) are isomers having C(8)H(10) . On oxidation (A) g...
Text Solution
|
- A hydrocarbon (A) of the formula , C7H12 on ozonolysis gives a compoun...
Text Solution
|
- On ozonolysis, an organic compound (A) C(6)H(10) gives two aldehyde (B...
Text Solution
|
- A compound A with molecular formula C(15)H(13)Cl gives a white precipi...
Text Solution
|
- An organic compound [A] C(6)H(10), on reduction first gives [B] C(6)H(...
Text Solution
|
- A hydrocarbon (A) of the formula C(7)H(12) on ozonolysis gives a compo...
Text Solution
|
- A compound 'X' on ozonolysis followed by reduction gives an aldehyde, ...
Text Solution
|
- Compound C(4)H(8)CI(2) (A) on hydrolysis gives a compound C(4)H(8)O(B)...
Text Solution
|