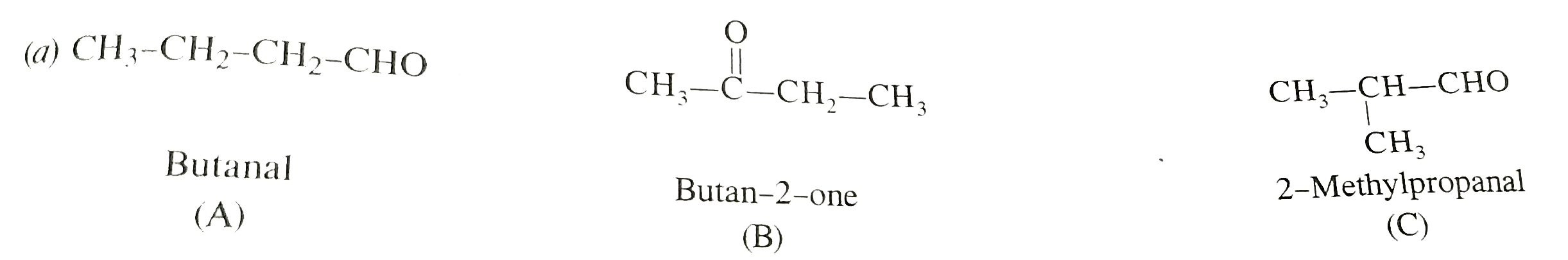Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (A), (B) and (C ) are three non-cylic funtional isomers of a carbonyl ...
Text Solution
|
- (A) A(C(7)H(14)) overset(O(3)//Red)(rarr)B(C(3)H(6)O)+C (B) Gives posi...
Text Solution
|
- (A) A(C(7)H(14)) overset(O(3)//Red)(rarr)B(C(3)H(6)O)+C (B) Gives posi...
Text Solution
|
- (A) A(C(7)H(14)) overset(O(3)//Red)(rarr)B(C(3)H(6)O)+C (B) Gives po...
Text Solution
|
- (A) A(C(7)H(14)) overset(O(3)//Red)(rarr)B(C(3)H(6)O)+C (B) Gives po...
Text Solution
|
- From the following sequence of reactions , [A] (C(6)H(12)) overset(H...
Text Solution
|
- From the following sequence of reactions , [A] (C(6)H(12)) overset(H...
Text Solution
|
- From the following sequence of reactions , [A] (C(6)H(12)) overset(H...
Text Solution
|
- Given are the isomers of C(8)H(8)O(2). Which isomer gives posi...
Text Solution
|