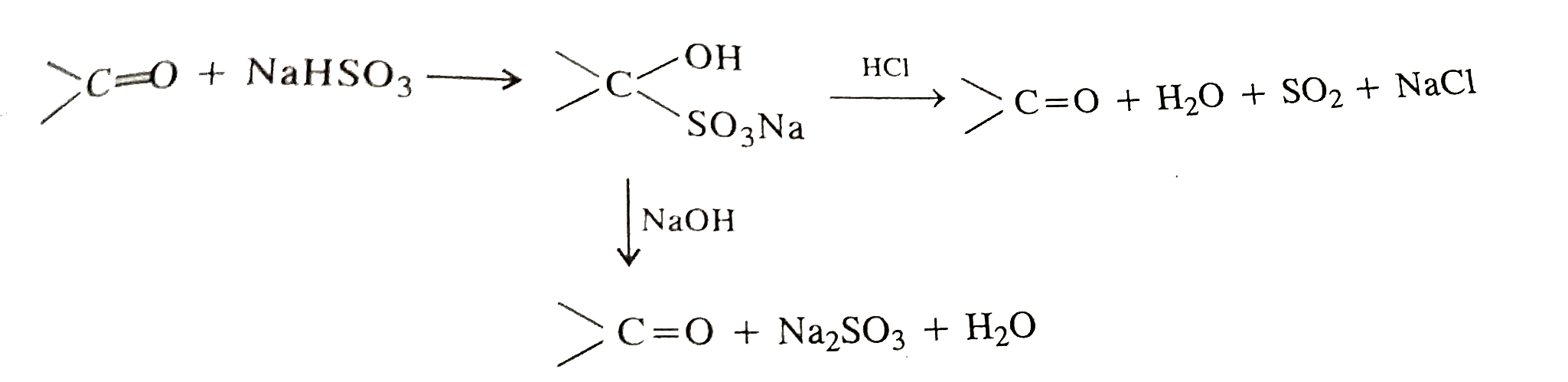Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Sodium bisulphite is used to purify aldehydes and ketones. Explain.
Text Solution
|
- Aldehydes and ketones are distinguished by using :
Text Solution
|
- Reduction of aldehydes and ketones into hydrocarbons using hydrazine a...
Text Solution
|
- Give suitable explanation for the following: (i) Chloral hydrate is ...
Text Solution
|
- Give reasons for the following : (iii) Sodium bisulphite is used for p...
Text Solution
|
- Give suitable explanation for the following: (i) Chloral hydrate is ...
Text Solution
|
- Most of ketones does not give addition compound with sodium bisulphite...
Text Solution
|
- ऐल्डिहाइडों तथा कीटोनों के शोधन में सोडियम बाइसल्फाइट का प्रयोग क्यों ...
Text Solution
|
- एल्डिहाइड और कीटोन के शुद्धिकरण के लिए सोडियम-बाइसल्फाइट (NaHSO3) का प...
Text Solution
|