Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
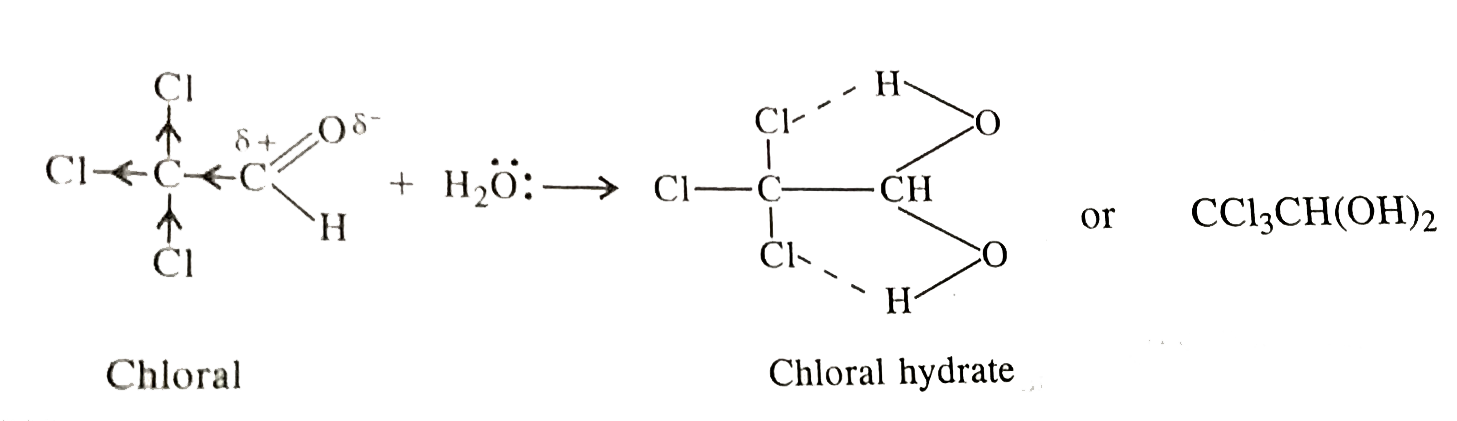
- Chloral hydrate is a gem-diol but still stable. How will you account f...
Text Solution
|
- Which of the following gem-diol is stable ? .
Text Solution
|
- Arrange their stabilities of given gem-diols in decreasing order .
Text Solution
|
- Assertion : Chloral hydrate is a stable compound. Reason : It is sta...
Text Solution
|
- Arrange the following gem diols in decreasing order of stability :
Text Solution
|
- Which of the following 'gem' diols is stable ?
Text Solution
|
- Some of the hydrated crystals are efflorescent . How do you account fo...
Text Solution
|
- Aldehydes usually do not form stable hydrates but chloral normally exi...
Text Solution
|
- সাধারণ অ্যালডিহাইডগুলি সুস্থিত হাইড্রেট গঠন করতে সমর্থ না হলেও ক্লোরাল...
Text Solution
|