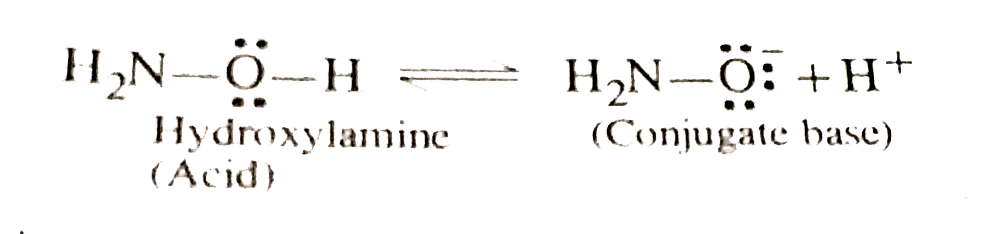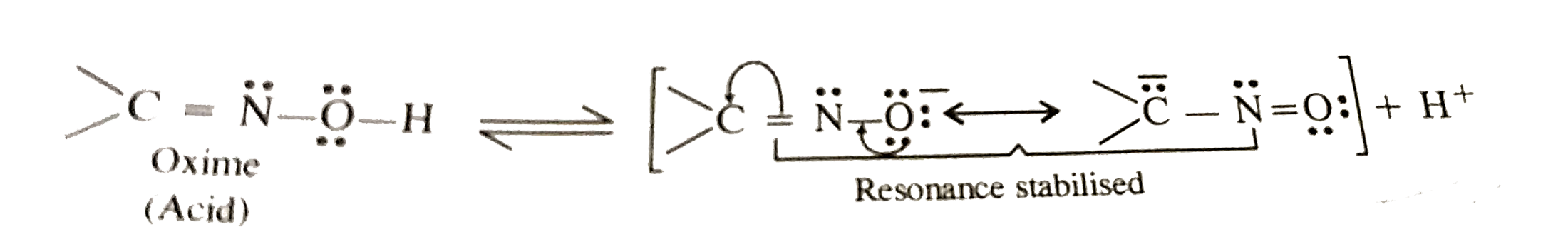Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compare th relative acidic strengths of hydroxyl amine and am oxime.
Text Solution
|
- Acetophenone on reaction with hydroxyl amine hydrochloride can produce...
Text Solution
|
- If the concentration of two monobasic acids are same, their relative s...
Text Solution
|
- Which of the following carbonyl compounds can give two oximes on react...
Text Solution
|
- Compare acidic strength of
Text Solution
|
- Assertion: Oximes are less acidic than hydroxyl amine. Reason: Oxim...
Text Solution
|
- ऑक्सीम, हाइड्रॉक्सिल ऐमीन से अधिक अम्लीय होती है, क्यो?
Text Solution
|
- Reduction of nitroalkane produces (1) 1^(@) -amines (2) N-alkyl hydrox...
Text Solution
|
- Which one of the following gives oxime with hydroxyl amine?
Text Solution
|
 group in their molecules and are expected to be weakly acidic. However, oxime is actually more acidic. Infact, hydroxyl amine after losing a proton forms a conjugate base in which negative charge is localised only on the oxygen atom.
group in their molecules and are expected to be weakly acidic. However, oxime is actually more acidic. Infact, hydroxyl amine after losing a proton forms a conjugate base in which negative charge is localised only on the oxygen atom.