Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
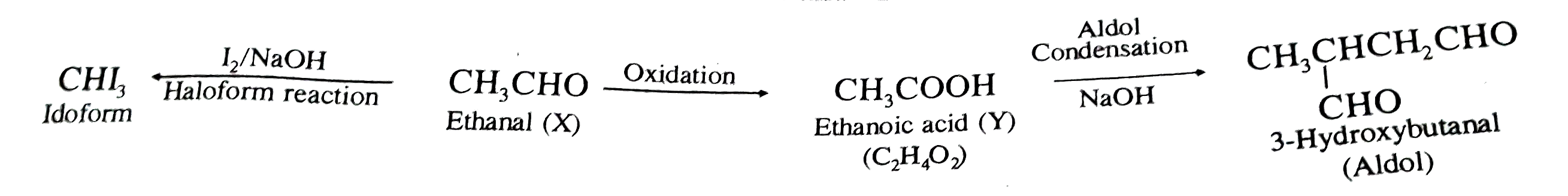
- A compound 'X' (C(2)H(4)O) on oxidation gives 'Y' (C(2)H(4)O(2)). The ...
Text Solution
|
- A compound X when passed through dil. H(2)SO(4) containing HgSO(4) giv...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive test with NaOH ...
Text Solution
|
- A compound 'X' (C(14)H(14)O) on mild oxidation yields C(14)H(12)(Y). I...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive teast with NaOH po...
Text Solution
|
- A compound 'X' on ozonolysis followed by reduction gives an aldehyde, ...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive test with NaOH and...
Text Solution
|
- एक यौगिक (X)(C2H4O) ऑक्सीकरण पर Y(C2H4O2) देता है । यौगिक 'X' हैलोफॉर्...
Text Solution
|
- An organic compound C(6))H(12)(X) on reduction gives C(6)H(14)(Y) . X ...
Text Solution
|