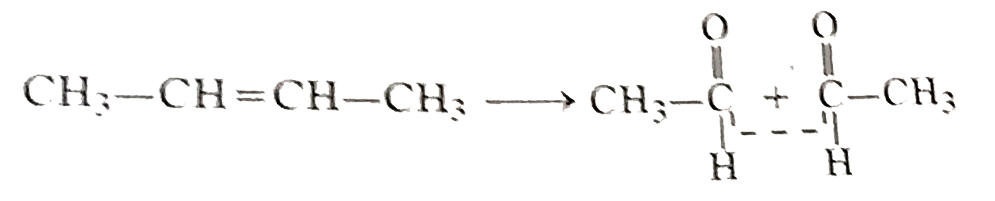Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of symmetrical alkene upon ozonolysis gives two moles of an a...
Text Solution
|
- One mole of symmetrical alkene on ozonolysis gives two moles of an ald...
Text Solution
|
- One mole of alkene an ozonolysis gives two moles of butanone. The alke...
Text Solution
|
- One mole of a symmetrical alkene on hydrolyses gives two moles of an a...
Text Solution
|
- One mole of a symmetrical alkene on ozonolysis gives two moles of an a...
Text Solution
|
- One mole of a symmetrical alkene on ozonolysis gives two of an aldehyd...
Text Solution
|
- One mole of a symmetrical alkene on ozonolysis gives two moles of an a...
Text Solution
|
- One mole of alkene on ozonolysis gives of butanone. The alkene is
Text Solution
|
- One mole of a symmetrical alkene on ozonolysis gives two moles of an a...
Text Solution
|