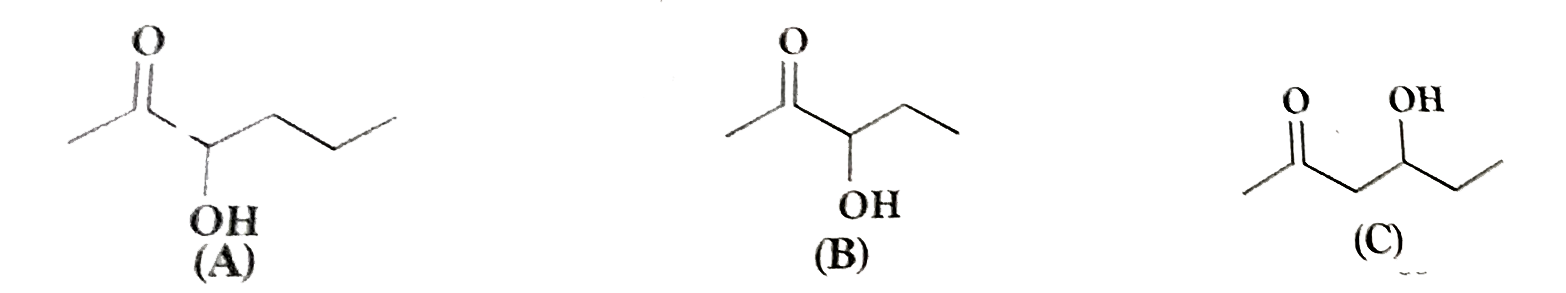
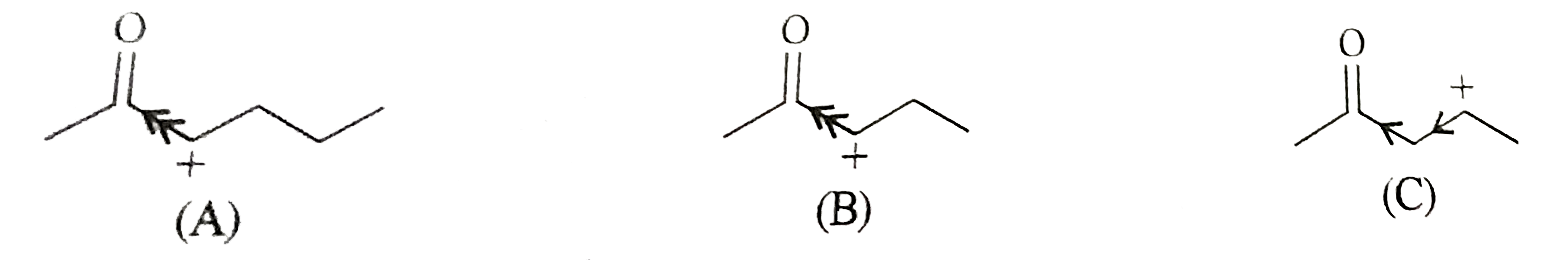
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Out of the following compounds, which will be dehydrated most readily?
Text Solution
|
- Which one of the following compounds will be most readily dehydrated?
Text Solution
|
- Which one of the following compounds will be most readily dehydrated?
Text Solution
|
- Which of the following would undergo dehydration most readily
Text Solution
|
- The compound that undergoes dehydration most readily is
Text Solution
|
- Which of the following will be dehydrated most readily in alkaline med...
Text Solution
|
- Which of the following compounds will be most readily dehydrated ?
Text Solution
|
- Which of the following will most readily dehydrated in acidic conditio...
Text Solution
|
- Which of the following will be dehydrated most readily in alkaline med...
Text Solution
|