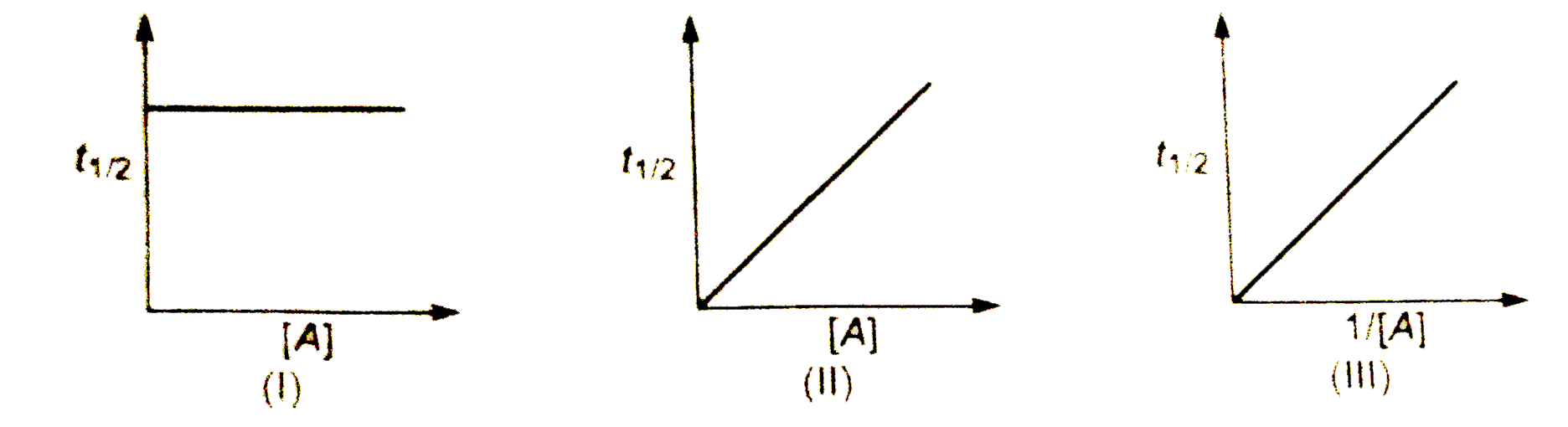
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the plots for the types of reaction nAtoB+C These plot...
Text Solution
|
- The correct nature of plot for first order reaction is (are):
Text Solution
|
- For a reaction nArarrB, following plots are given: The order of ...
Text Solution
|
- Consider the plots for the types of reaction nAtoB+C These plot...
Text Solution
|
- Consider the plots for the types of reaction nAtoB+C These plots respe...
Text Solution
|
- Which concentration plot is linear for a first order reaction?
Text Solution
|
- in the following plot for a first order reaction slope is equal to
Text Solution
|
- plot a graph of reaction -rate vs concentration of the reactant for a ...
Text Solution
|
- The given plots represent the variation of the concentration of a reac...
Text Solution
|