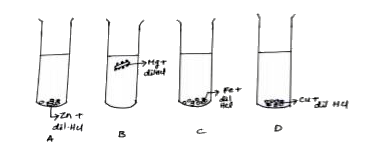
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
SAMPLE PAPER 2022
CBSE MODEL PAPER|Exercise SECTION - C|8 VideosSAMPLE PAPER 2022
CBSE MODEL PAPER|Exercise ALTERNATIVE QUESTIONS(SECTION A)|3 VideosSAMPLE PAPER 2022
CBSE MODEL PAPER|Exercise ALTERNATIVE QUESTIONS(SECTION B)|3 VideosAdditional Practice Questions
CBSE MODEL PAPER|Exercise Question|16 VideosSAMPLE PAPER 2022 TERM II
CBSE MODEL PAPER|Exercise SECTION B|4 Videos
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-SAMPLE PAPER 2022-SECTION - B
- Identify the correct option from the given table which represents the ...
Text Solution
|
- In which year is concentration of hydrogen ion the highest?
Text Solution
|
- The diagram shows the reaction between metal and dil. acid. What ...
Text Solution
|
- The table shown below gives information about four substances: A, B, C...
Text Solution
|
- Vinay observed that the stain of curry on a white shirt becomes reddis...
Text Solution
|
- In which of the following setups would the bulb glow?
Text Solution
|
- Assertion: Fresh milk in which baking soda is added, takes a longer ti...
Text Solution
|
- The table given below shows the reaction of a few elements with acids ...
Text Solution
|
- The table given below shows the reaction of a few elements with acids ...
Text Solution
|