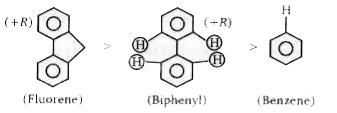
The electron distribution in benzene is symmetrical and a pair of electrons become localised at the requirement of the electrophilic reagent. In biphenyl, one ring behaves as a +R group, thereby activating the other ring in the o- and p-positions. Hence, on the charge distribution approach, biphenyl can be expected to be more reactive than benzene towards electrophiles. The +R effect in biphenyl is transmitted from one ring to the other through the intramolecular bond. Maximum transmission is obtained if the two rings are coplanar, but because of the steric effects (weak) of the hydrogens in the o-positions, the two rings are being hindered from becoming coplanar. At the same time, biphenyl molecules in solution are subject to thermal agitation (.bombardment by other molecules), and this also tends to prevent the biphenyl molecule from becoming coplanar (in which state the molecule will have a minimum entropy). The overall result is that the resonance effect is partially nullified, i.e., the activation of one ring by the other through resonance is relatively weak. If we now consider fluorene, its unsaturated system is the same as that of biphenyl, but in the former, because it is a flat molecule due to the presence of the middle ring (formed by the methylene bridge), the +R effect of one benzene ring is completely transmitted to the other ring. Hence, fluorene can be expected to be more reactive than biphenyl towards electrophiles. If we consider the problern from the carbonium - ion stability approach, then the more extended the conjugation and the more nearly coplanar the resonating structures, the more stable are the resonance hybrids. We therefore arrive at the same order of reactivity as before by using the same arguments.