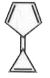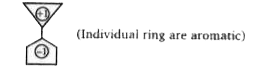A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MS CHOUHAN-AROMATIC COMPOUNDS -LEVEL-2 (SUBJECTIVE PROBLEMS)
- Caliene, C7 H6 , is expected to be a fairly polar aromatic molecule. W...
Text Solution
|
- Double bond equivalent of D is :
Text Solution
|
- How many isomers 'x' of C8H10 when reacts with hot alkaline KMnO4 gi...
Text Solution
|
- How many groups are op director in the electrophilic aromatic substitu...
Text Solution
|