A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MS CHOUHAN-PRACTICAL ORGANIC CHEMISTRY-LEVEL -2
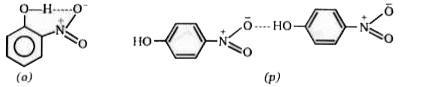
- A mixture of o-nitrophenol and p-nitrophenol can be separated by
Text Solution
|
- Which isomer gives positive iodoform test?
Text Solution
|
- Which isomer gives +ive Tollen's test, also reacts with FeCl(3)?
Text Solution
|
- Which isomer reacts with NaHCO(3)?
Text Solution
|
- Which isomer on hydrolysis gives 1,4-di hydroxybenzene?
Text Solution
|
- Ph-overset(O)overset(||)(C)-Ohoverset(14)overset(NaHCO(3))(to)(A)"gas"...
Text Solution
|