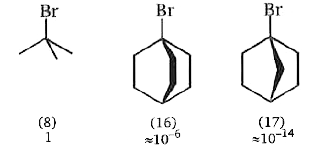
The effect of structure on relative reactivity may be seen particularly clearly when a halogen atom is located at the bridgehead of a bicyclic system. Thus the following rates were observed for solvolysis in 80% aqueous ethanol at `25^@`
All are tertiaryhalides so that attack by the `S_(N^2)` mode would notbe expected to occur on (16) or (17) any more than it did on (8). `S_(N^2)` attack .from the back. on the carbon atom carrying Br would in any case be prevented in (16) and (17) both sterically by their cagelike structure, and also by the impossibility of forcing their fairly rigid framework through transition states with the required planardistribution ofbonds to the bridgehead carbon atom. Solvolysis via rate- limiting formation ofthe ion pair `(S_(N^1))` as happens with (8) is also inhibited because the resultant carbocations from (16) and (17) would be unable, because of their rigid frameworks, to stabilise themselves by collapsing to the stable planar state. These carbocation intermediates are thus ofvery much higher energy level than usual, and therefore are formed only slowly and with reluctance. The very greatly reduced solvolysis rate of (17) compared with (16) reflects the greater rigidity about the bridgehead (cationic) carbon with a one carbon (17), than with a two- carbon (16), bridge.