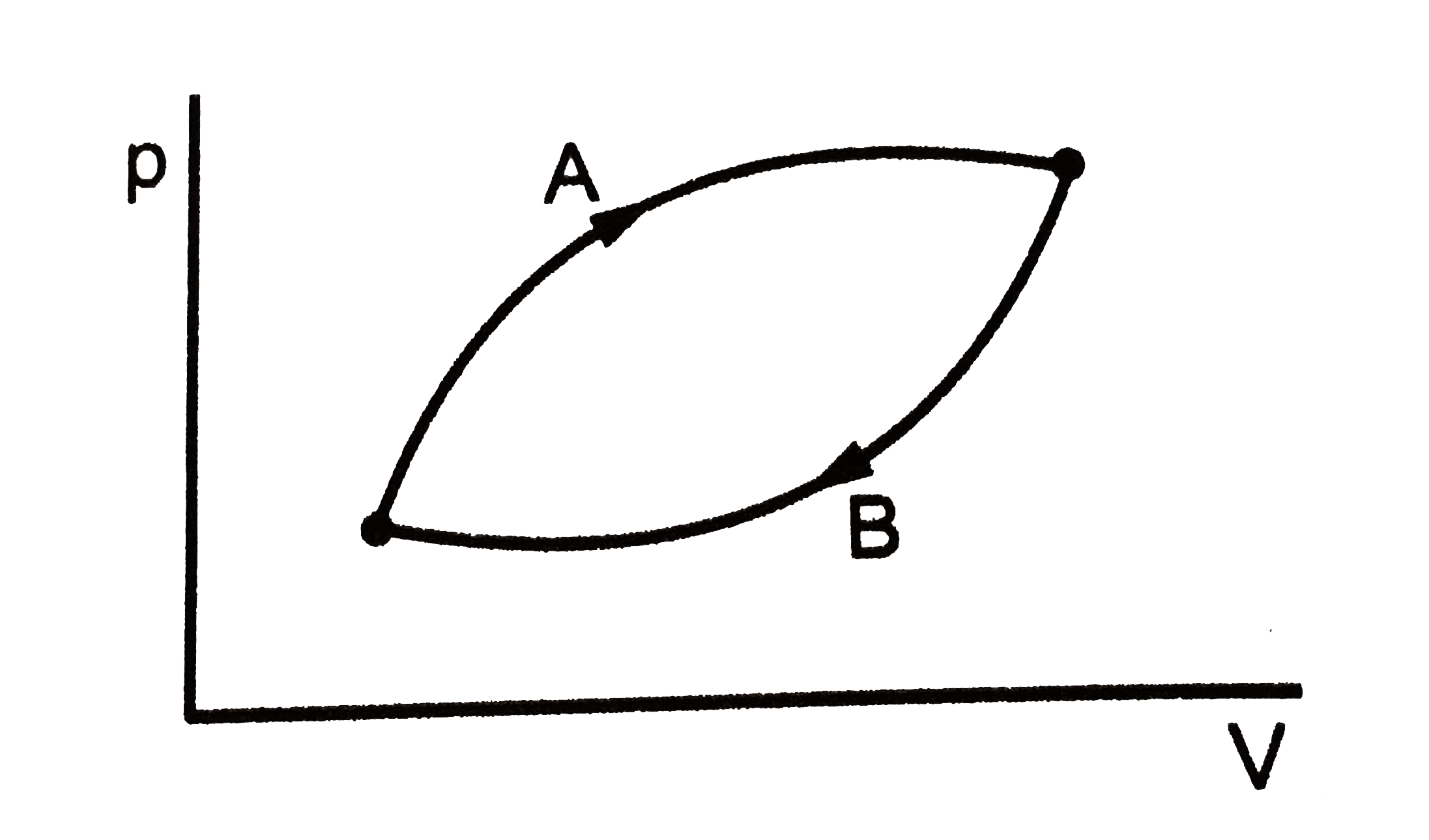
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
HC VERMA|Exercise Exercises|22 VideosLAWS OF THERMODYNAMICS
HC VERMA|Exercise Short Answer|14 VideosLAWS OF THERMODYNAMICS
HC VERMA|Exercise Objective I|9 VideosKINETIC THEORY OF GASES
HC VERMA|Exercise All Questions|120 VideosNEWTON'S LAWS OF MOTION
HC VERMA|Exercise Exercises|42 Videos
Similar Questions
Explore conceptually related problems
HC VERMA-LAWS OF THERMODYNAMICS-Objective II
- The pressure p and volume V of an ideal gas both increase in a process...
Text Solution
|
- a) In a process on a system, the initial pressure and volume are equal...
Text Solution
|
- A system can be taken from the initial state p(1),V(1) to the final st...
Text Solution
|
- Refer to figure. Let DeltaU(1) and DeltaU(2) be the changes in interna...
Text Solution
|
- The internal enegy of an ideal gas decreases by the same amount as the...
Text Solution
|