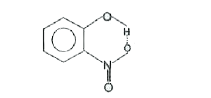
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise Recent Examination Questions |12 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise Multiple Choice Questions Level - II|80 VideosALDEHYDES , KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise Multiple Choice Questions ( Level - III )|10 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALCOHOLS, PHENOLS AND ETHERS -Multiple Choice Questions Level - III
- The major product obtained on interaction of phenol with NaOH and CO(2...
Text Solution
|
- Phenol is heated with a solution of mixture of KBr and KBrO(3). The ma...
Text Solution
|
- The correct order of acid strength of the following compounds : (I) ...
Text Solution
|
- Which of the following reagents may be used to distinguish between phe...
Text Solution
|
- Ortho- nitrophenol is less soluble in water than p-and m-nitrophenols ...
Text Solution
|
- Arrange the following compounds in order of decreasing acidity :
Text Solution
|
- An unknown alcohol is treated with the 'Lucas reagent' to determine wh...
Text Solution
|
- Sodium phenoxide when heated with CO(2) under pressure at 125^(@)C yie...
Text Solution
|