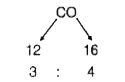
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION JHARKHAND-LAWS OF CHEMICAL COMBINATION AND GAS LAWS -Exam Booster for Cracking Exam
- 8 g of oxygen combine with 1 g of hydrogen and 29 g of calcium therefo...
Text Solution
|
- If 2.0 g of the hydrogen on burning in 16.0 g of oxygen forms 18.0 g o...
Text Solution
|
- The ratio in weight by which carbon and oxygen combine in a molecule o...
Text Solution
|
- Which of the following statements are correct? (I) When water is de...
Text Solution
|
- A gaseous mixture contains H(2) , and N(2) , in the ratio of 1:4 by we...
Text Solution
|
- Real gas will approach the behaviour of ideal gas at
Text Solution
|
- Nitrogen combines, with oxygen to form five gaseous oxides N(2)O, NO, ...
Text Solution
|
- Equal volumes of all gases under same temperature and pressure contain...
Text Solution
|
- Matter can be converted into energy. It is represented by
Text Solution
|
- Which of the following is not a chemical reaction?
Text Solution
|
- The law regarding the conversion of mass into energy was given by
Text Solution
|
- The law of multiple proportions is illustrated by the two compounds
Text Solution
|
- If the pressure of a gas is reduced to half and temperature is doubled...
Text Solution
|
- Sparking is produced in a mixture 20 mL O(2) , and 20 mL CO, the volum...
Text Solution
|
- A 500 mL flask contains 400 mL H(2), at 700 mm, 200 mL N(2), at 350 mm...
Text Solution
|
- Hydrogen diffuses 6 times faster than the gas X, the molecular weight ...
Text Solution
|
- Which is correct relation between rate of diffusion and their densitie...
Text Solution
|
- At constant pressure the volume of a gas is Vat 0^(@) C. At which temp...
Text Solution
|
- A sample of nitrogen gas occupies a volume of 320 mL at STP calculate ...
Text Solution
|
- Equal weights of methane and oxygen are mixed in a vessel. The partial...
Text Solution
|