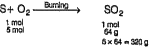A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION JHARKHAND-CONCEPT OF ATOMIC MOLECULAR AND EQUIVALENT MASSES-EXAM BOOSTER FOR CRACKING EXAM
- 19.7 kg of gold was recovered from a smuggler. How many atoms of gold ...
Text Solution
|
- Equivalent weight of sulphur in SCl2 is 16. What is the equivalent we...
Text Solution
|
- What weight of SO(2) can be made by burning sulphur in 5.0 moles of ox...
Text Solution
|
- Equivalent weight of a metal is 29.4. It forms metal sulphate isomorph...
Text Solution
|
- 2 g of oxygen contain number of atoms equal to that contained in
Text Solution
|
- Number of atoms in 4.25 g of NH3 is (approx.)
Text Solution
|
- If the density of water is 1 g cm^2 , then the volume occupied by one ...
Text Solution
|
- The equivalent weight of potassium chromate as an oxidising agent in a...
Text Solution
|
- In the reaction 2Na2S2O3 + I2 to Na2S4O6 + 2NaI The equivalent...
Text Solution
|
- The equivalent weight of MnSO4 is half its molecular weight when it i...
Text Solution
|
- 2240 mL of NH3 gas at NTP weight
Text Solution
|
- At NTP 5.6 L of gas has a mass of 60 g. The vapour density of the gas ...
Text Solution
|
- Which of the following has largest number of atoms?
Text Solution
|
- The number of moles of CO2 which contain 16 g of oxygen is
Text Solution
|
- Which of the following sets of compounds have their molecular weight a...
Text Solution
|
- 1 g of metal oxide on reduction gives 0.68 g of the metal. The equival...
Text Solution
|
- The weight of oxalic acid dihydrate is 126. Its equivalent weight is
Text Solution
|
- Equivalent weight of nitrogen varies in its oxides, because it
Text Solution
|
- The determination of vapour density of a substance is useful to determ...
Text Solution
|
- A mole of compound is composed of 6.023 xx 10^23 atoms of hydrogen, 3...
Text Solution
|