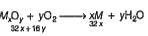A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION JHARKHAND-CONCEPT OF ATOMIC MOLECULAR AND EQUIVALENT MASSES-EXAM BOOSTER FOR CRACKING EXAM
- Which of the following sets of compounds have their molecular weight a...
Text Solution
|
- 1 g of metal oxide on reduction gives 0.68 g of the metal. The equival...
Text Solution
|
- The weight of oxalic acid dihydrate is 126. Its equivalent weight is
Text Solution
|
- Equivalent weight of nitrogen varies in its oxides, because it
Text Solution
|
- The determination of vapour density of a substance is useful to determ...
Text Solution
|
- A mole of compound is composed of 6.023 xx 10^23 atoms of hydrogen, 3...
Text Solution
|
- 0.45 g of acid (molecular weight 90) was neutralised by 20 mL 0.5 N ca...
Text Solution
|
- The amount of zinc for getting 336 mL H2 from an acid at NTP will be ...
Text Solution
|
- 4 g of metal oxide MxOy is reduced by H2 and 2.4 g metal is obtained,...
Text Solution
|
- In Victor Meyer method 0.45 g of a volatile liquid displace 112 cc air...
Text Solution
|
- A metal oxide contains 32% oxygen and the vapour density of its chlori...
Text Solution
|
- Chloride MCl2 of a metal M contains 52.07% chlorine. Its atomic weigh...
Text Solution
|
- 1.60 g of metal oxide on heating with H, gives 0.86 g water. The equiv...
Text Solution
|
- The number of molecules in 19.75 g KMnO4 is (K= 39, Mn= 55, O=16)
Text Solution
|
- Haemoglobin is a compound of iron. It contains 0.335% iron. If one mol...
Text Solution
|
- 26 g carbon is burnt and CO2 formed is absorbed in soda lime and ther...
Text Solution
|
- The specific heat of an element is 0.031 cal/g/""^@C. 25.9 g of it rea...
Text Solution
|
- The weight of 56 cc of, a gas at NTP is 0.11 g. Its molecular weight i...
Text Solution
|
- Which of the following will contain same number of atoms as 20g of cal...
Text Solution
|
- A metal chloride contains 25.26% metal. Its equivalent weight is
Text Solution
|